Cell clusters overlying focally disrupted mammary myoepithelial cell layers and adjacent cells within the same duct display different immunohistochemical and genetic features: implications for tumor progression and invasion
- PMID: 14580259
- PMCID: PMC314413
- DOI: 10.1186/bcr653
Cell clusters overlying focally disrupted mammary myoepithelial cell layers and adjacent cells within the same duct display different immunohistochemical and genetic features: implications for tumor progression and invasion
Abstract
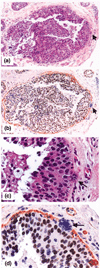
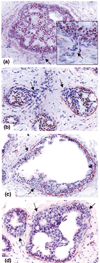
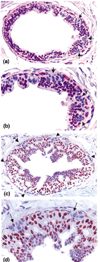
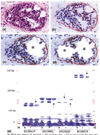
Introduction: Our previous studies detected focal disruptions in myoepithelial cell layers of several ducts with carcinoma in situ. The cell cluster overlying each of the myoepithelial disruptions showed a marked reduction in or a total loss of immunoreactivity for the estrogen receptor (ER). This is in contrast to the adjacent cells within the same duct, which were strongly immunoreactive for the ER. The current study attempts to confirm and expand previous observations on a larger scale.
Methods: Paraffin sections from 220 patients with ER-positive intraductal breast tumors were double immunostained with the same protocol previously used. Cross-sections of ducts lined by > or = 40 epithelial cells were examined for myoepithelial cell layer disruptions and for ER expression. In five selected cases, ER-negative cells overlying the disrupted myoepithelial cell layer and adjacent ER-positive cells within the same duct were separately microdissected and assessed for loss of heterozygosity and microsatellite instability.
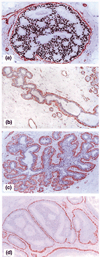
Results: Of the 220 cases with 5698 duct cross-sections examined, 94 showed disrupted myoepithelial cell layers with 405 focal disruptions. Of the 94 cases, 79 (84%) contained only ER-negative cell clusters, nine (9.6%) contained both ER-negative and ER-positive cell clusters, and six (6.4%) contained only ER-positive cell clusters overlying disrupted myoepithelial cell layers. Of the 405 disruptions, 350 (86.4%) were overlain by ER-negative cell clusters and 55 (13.6%) were overlain by ER-positive cell clusters (P < 0.01). Microdissected ER-negative and ER-positive cells within the same duct from all five selected cases displayed a different frequency or pattern of loss of heterozygosity and/or microsatellite instability at 10 of the 15 DNA markers.
Conclusions: Cells overlying focally disrupted myoepithelial layers and their adjacent counterparts within the same duct displayed different immunohistochemical and molecular features. These features potentially represent an early sign of the formation of a biologically more aggressive cell clone and the myoepithelial cell layer breakdown possibly associated with tumor progression or invasion.
Figures


Similar articles
-
Atypical E-cadherin expression in cell clusters overlying focally disrupted mammary myoepithelial cell layers: implications for tumor cell motility and invasion.Pathol Res Pract. 2009;205(6):375-85. doi: 10.1016/j.prp.2008.08.009. Epub 2009 Apr 22. Pathol Res Pract. 2009. PMID: 19395181
-
A subset of in situ breast tumor cell clusters lacks expression of proliferation and progression related markers but shows signs of stromal and vascular invasion.Cancer Detect Prev. 2005;29(4):323-31. doi: 10.1016/j.cdp.2005.06.010. Cancer Detect Prev. 2005. PMID: 16122886
-
Mammary ducts with and without focal myoepithelial cell layer disruptions show a different frequency of white blood cell infiltration and growth pattern: implications for tumor progression and invasion.Appl Immunohistochem Mol Morphol. 2005 Mar;13(1):30-7. doi: 10.1097/00129039-200503000-00006. Appl Immunohistochem Mol Morphol. 2005. PMID: 15722791
-
The significance of focal myoepithelial cell layer disruptions in human breast tumor invasion: a paradigm shift from the "protease-centered" hypothesis.Exp Cell Res. 2004 Dec 10;301(2):103-18. doi: 10.1016/j.yexcr.2004.08.037. Exp Cell Res. 2004. PMID: 15530847 Review.
-
Carcinoma in situ of the female breast. A clinico-pathological, immunohistological, and DNA ploidy study.APMIS Suppl. 2003;(108):1-67. APMIS Suppl. 2003. PMID: 12874968 Review.
Cited by
-
Tumor cell budding from focally disrupted tumor capsules: a common pathway for all breast cancer subtype derived invasion?J Cancer. 2010 Jun 2;1:32-7. doi: 10.7150/jca.1.32. J Cancer. 2010. PMID: 20842222 Free PMC article.
-
Versican expression in canine carcinomas in benign mixed tumours: is there an association with clinical pathological factors, invasion and overall survival?BMC Vet Res. 2012 Oct 20;8:195. doi: 10.1186/1746-6148-8-195. BMC Vet Res. 2012. PMID: 23082892 Free PMC article.
-
Alteration in protein expression in estrogen receptor alpha-negative human breast cancer tissues indicates a malignant and metastatic phenotype.Clin Exp Metastasis. 2010 Oct;27(7):493-503. doi: 10.1007/s10585-010-9338-8. Epub 2010 Jul 3. Clin Exp Metastasis. 2010. PMID: 20602252 Free PMC article.
-
Rare co-existence of multifocal myoepitheliosis with infiltrating ductal carcinoma of the breast.J Clin Pathol. 2007 Sep;60(9):1057-8. doi: 10.1136/jcp.2006.042531. J Clin Pathol. 2007. PMID: 17761742 Free PMC article. No abstract available.
-
Novel stromal biomarkers in human breast cancer tissues provide evidence for the more malignant phenotype of estrogen receptor-negative tumors.J Biomed Biotechnol. 2011;2011:723650. doi: 10.1155/2011/723650. Epub 2011 Oct 3. J Biomed Biotechnol. 2011. PMID: 21976967 Free PMC article.
References
-
- Tsubura A, Shikata N, Inui T, Morii S, Hatano T, Oikawa T. Immunohistochemical localization of myoepithelial cells and basement membrane in normal, benign and malignant human breast lesions. Virchows Arch. 1988;413:133–139. - PubMed
-
- Jolicoeur F, Seemayer TA, Gabbiani G, Robidoux A, Gaboury L, Oligny LL, Schurch W. Multifocal, nascent, and invasive myoepithelial carcinoma (malignant myoepithelioma) of the breast: an immunohistochemical and ultrastructural study. Int J Surg Pathol. 2002;10:281–291. - PubMed
-
- Slade MJ, Coope RC, Gomm JJ, Coombes RC. The human mammary gland basement membrane is integral to the polarity of luminal epithelial cells. Exp Cell Res. 1999;247:267–278. - PubMed
-
- Miosge N. The ultrastructural composition of basement membrane in vivo. Histol Histopathol. 2001;16:1239–1248. - PubMed
-
- Nerlich A. Morphology of basement membrane and associated matrix proteins in normal and pathological tissues. Veroff Pathol. 1995;145:1–139. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

