Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol
- PMID: 14581208
- PMCID: PMC1303584
- DOI: 10.1016/S0006-3495(03)74726-2
Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol
Abstract
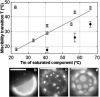
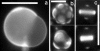
We use fluorescence microscopy to directly observe liquid phases in giant unilamellar vesicles. We find that a long list of ternary mixtures of high melting temperature (saturated) lipids, low melting temperature (usually unsaturated) lipids, and cholesterol produce liquid domains. For one model mixture in particular, DPPC/DOPC/Chol, we have mapped phase boundaries for the full ternary system. For this mixture we observe two coexisting liquid phases over a wide range of lipid composition and temperature, with one phase rich in the unsaturated lipid and the other rich in the saturated lipid and cholesterol. We find a simple relationship between chain melting temperature and miscibility transition temperature that holds for both phosphatidylcholine and sphingomyelin lipids. We experimentally cross miscibility boundaries both by changing temperature and by the depletion of cholesterol with beta-cyclodextrin. Liquid domains in vesicles exhibit interesting behavior: they collide and coalesce, can finger into stripes, and can bulge out of the vesicle. To date, we have not observed macroscopic separation of liquid phases in only binary lipid mixtures.
Figures
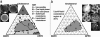



References
-
- Anderson, R. G., and K. Jacobson. 2002. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 296:1821–1825. - PubMed
-
- Angelova, M. I., S. Soleau, P. Meleard, J. F. Faucon, and P. Bothorel. 1992. Preparation of giant vesicles by external AC electric fields. Prog. Colloid Polym. Sci. 89:127–131.
-
- Arneodo, A., Y. Couder, G. Grasseau, V. Hakim, and M. Rabaud. 1990. Pattern growth: from smooth interfaces to fractal structures. In Nonlinear Evolution of Spatio-Temporal Structures in Dissipative Continuous Systems. F. H. Busse and L. Kramer, editors. Plenum Press, New York. 481.
-
- Bacha, D., and E. Wachtel. 2003. Phospholipid/cholesterol model membranes: formation of cholesterol crystallites. Biochim. Biophys. Acta. 1610:187–197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

