Vps27-Hse1 and ESCRT-I complexes cooperate to increase efficiency of sorting ubiquitinated proteins at the endosome
- PMID: 14581452
- PMCID: PMC2173515
- DOI: 10.1083/jcb.200305007
Vps27-Hse1 and ESCRT-I complexes cooperate to increase efficiency of sorting ubiquitinated proteins at the endosome
Abstract
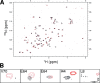
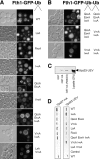
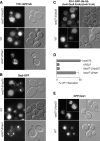
Ubiquitin (Ub) attachment to cell surface proteins causes their lysosomal degradation by incorporating them into lumenal membranes of multivesicular bodies (MVBs). Two yeast endosomal protein complexes have been proposed as Ub-sorting "receptors," the Vps27-Hse1 complex and the ESCRT-I complex. We used NMR spectroscopy and mutagenesis studies to map the Ub-binding surface for Vps27 and Vps23. Mutations in Ub that ablate only Vps27 binding or Vps23 binding blocked the ability of Ub to serve as an MVB sorting signal, supporting the idea that both the Vps27-Hse1 and ESCRT-I complexes interact with ubiquitinated cargo. Vps27 also bound Vps23 directly via two PSDP motifs present within the Vps27 COOH terminus. Loss of Vps27-Vps23 association led to less efficient sorting into the endosomal lumen. However, sorting of vacuolar proteases or the overall biogenesis of the MVB were not grossly affected. In contrast, disrupting interaction between Vps27 and Hse1 caused severe defects in carboxy peptidase Y sorting and MVB formation. These results indicate that both Ub-sorting complexes are coupled for efficient recognition of ubiquitinated cargo.
Figures



References
-
- Babst, M., G. Odorizzi, E.J. Estepa, and S.D. Emr. 2000. Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic. 1:248–258. - PubMed
-
- Babst, M., D.J. Katzmann, W.B. Snyder, B. Wendland, and S.D. Emr. 2002. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell. 3:283–289. - PubMed
-
- Bache, K.G., C. Raiborg, A. Mehlum, and H. Stenmark. 2003. STAM and Hrs are subunits of a multivalent ubiquitin-binding complex on early endosomes. J. Biol. Chem. 278:12513–12521. - PubMed
-
- Bilodeau, P.S., J.L. Urbanowski, S.C. Winistorfer, and R.C. Piper. 2002. The Vps27p Hse1p complex binds ubiquitin and mediates endosomal protein sorting. Nat. Cell Biol. 4:534–539. - PubMed
-
- Carter, C.A. 2002. Tsg101: HIV-1's ticket to ride. Trends Microbiol. 10:203–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

