Integrin-linked kinase is required for laminin-2-induced oligodendrocyte cell spreading and CNS myelination
- PMID: 14581460
- PMCID: PMC2173507
- DOI: 10.1083/jcb.200304154
Integrin-linked kinase is required for laminin-2-induced oligodendrocyte cell spreading and CNS myelination
Erratum in
- J Cell Biol. 2006 Jul 17;174(2):315
Abstract
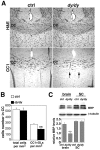
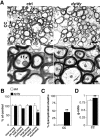
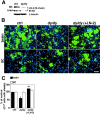
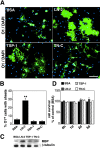
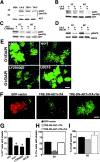
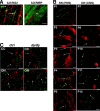
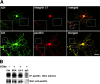
Early steps in myelination in the central nervous system (CNS) include a specialized and extreme form of cell spreading in which oligodendrocytes extend large lamellae that spiral around axons to form myelin. Recent studies have demonstrated that laminin-2 (LN-2; alpha2beta1gamma1) stimulates oligodendrocytes to extend elaborate membrane sheets in vitro (cell spreading), mediated by integrin alpha6beta1. Although a congenital LN-2 deficiency in humans is associated with CNS white matter changes, LN-2-deficient (dy/dy) mice have shown abnormalities primarily within the peripheral nervous system. Here, we demonstrate a critical role for LN-2 in CNS myelination by showing that dy/dy mice have quantitative and morphologic defects in CNS myelin. We have defined the molecular pathway through which LN-2 signals oligodendrocyte cell spreading by demonstrating requirements for phosphoinositide 3-kinase activity and integrin-linked kinase (ILK). Interaction of oligodendrocytes with LN-2 stimulates ILK activity. A dominant negative ILK inhibits LN-2-induced myelinlike membrane formation. A critical component of the myelination signaling cascade includes LN-2 and integrin signals through ILK.
Figures


References
-
- Back, S.A., R. Khan, X. Gan, P.A. Rosenberg, and J.J. Volpe. 1999. A new Alamar blue viability assay to rapidly quantify oligodendrocyte death. J. Neurosci. Methods. 91:47–54. - PubMed
-
- Bjartmar, C., C. Hildebrand, and K. Loinder. 1994. Morphological heterogeneity of rat oligodendrocytes: electron microscopic studies on serial sections. Glia. 11:235–244. - PubMed
-
- Bradley, W.G., and E. Jaros. 1979. Involvement of peripheral and central nerves in murine dystrophy. Ann NY Acad Sci. 317:132–142. - PubMed
-
- Buttery, P.C., and C. ffrench-Constant. 1999. Laminin-2/integrin interactions enhance myelin membrane formation by oligodendrocytes. Mol. Cell. Neurosci. 14:199–212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

