Human immunodeficiency virus type 1 enters primary human brain microvascular endothelial cells by a mechanism involving cell surface proteoglycans independent of lipid rafts
- PMID: 14581551
- PMCID: PMC254292
- DOI: 10.1128/jvi.77.22.12140-12151.2003
Human immunodeficiency virus type 1 enters primary human brain microvascular endothelial cells by a mechanism involving cell surface proteoglycans independent of lipid rafts
Abstract
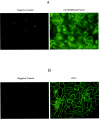
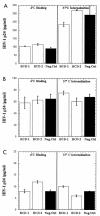
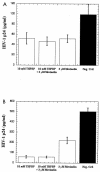
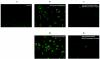
Several studies have reported a crucial role for cholesterol-enriched membrane lipid rafts and cell-associated heparan sulfate proteoglycans (HSPGs), a class of molecules that can localize in lipid rafts, in the entry of human immunodeficiency virus type 1 (HIV-1) into permissive cells. For the present study, we examined the role of these cell surface moieties in HIV-1 entry into primary human brain microvascular endothelial cells (BMVECs), which represent an important HIV-1 central nervous system-based cell reservoir and a portal for neuroinvasion. Cellular cholesterol was depleted by exposure to beta-cyclodextrins and 3-hydroxy-3-methylglutaryl (HMG)-coenzyme A reductase inhibitors (statins), the loss of cholesterol was quantitated, and disruption of membrane rafts was verified by immunofluorescence. Nevertheless, these treatments did not affect binding of several strains of HIV-1 virions to BMVECs at 4 degrees C or their infectivities at 37 degrees C. In contrast, we confirmed that cholesterol depletion and raft disruption strongly inhibited HIV-1 binding and infection of Jurkat T cells. Enzymatic digestion of cell-associated HSPGs on human BMVECs dramatically inhibited HIV-1 infection, and our data from quantitative HIV-1 DNA PCR analysis strongly suggest that cell-associated chondroitin sulfate proteoglycans greatly facilitate infective entry of HIV-1 into human BMVECs. These findings, in combination with our earlier work showing that human BMVECs lack CD4, indicate that the molecular mechanisms for HIV-1 entry into BMVECs are fundamentally different from that of viral entry into T cells, in which lipid rafts, CD4, and probably HSPGs play important roles.
Figures




References
-
- Allain, C. C., L. S. Poon, C. S. Chan, W. Richmond, and P. C. Fu. 1974. Enzymatic determination of total serum cholesterol. Clin. Chem. 20:470-475. - PubMed
-
- An, S. F., M. Groves, F. Gray, and F. Scaravilli. 1999. Early entry and widespread cellular involvement of HIV-1 DNA in brains of HIV-1 positive asymptomatic individuals. J. Neuropathol. Exp. Neurol. 58:1156-1162. - PubMed
-
- Anderson, R. G., and K. Jacobson. 2002. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science 296:1821-1825. - PubMed
-
- Bagasra, O., E. Lavi, L. Bobroski, K. Khalili, J. P. Pestaner, R. Tawadros, and R. J. Pomerantz. 1996. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS 10:573-585. - PubMed
-
- Bell, J. E., A. Busuttil, J. W. Ironside, S. Rebus, Y. K. Donaldson, P. Simmonds, and J. F. Peutherer. 1993. Human immunodeficiency virus and the brain: investigation of virus load and neuropathologic changes in pre-AIDS subjects. J. Infect. Dis. 168:818-824. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

