Voltage-dependent gating of the cystic fibrosis transmembrane conductance regulator Cl- channel
- PMID: 14581585
- PMCID: PMC2229579
- DOI: 10.1085/jgp.200308921
Voltage-dependent gating of the cystic fibrosis transmembrane conductance regulator Cl- channel
Abstract
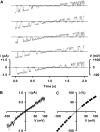
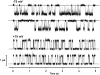
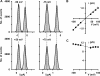
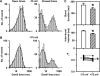
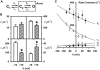
When excised inside-out membrane patches are bathed in symmetrical Cl--rich solutions, the current-voltage (I-V) relationship of macroscopic cystic fibrosis transmembrane conductance regulator (CFTR) Cl- currents inwardly rectifies at large positive voltages. To investigate the mechanism of inward rectification, we studied CFTR Cl- channels in excised inside-out membrane patches from cells expressing wild-type human and murine CFTR using voltage-ramp and -step protocols. Using a voltage-ramp protocol, the magnitude of human CFTR Cl- current at +100 mV was 74 +/- 2% (n = 10) of that at -100 mV. This rectification of macroscopic CFTR Cl- current was reproduced in full by ensemble currents generated by averaging single-channel currents elicited by an identical voltage-ramp protocol. However, using a voltage-step protocol the single-channel current amplitude (i) of human CFTR at +100 mV was 88 +/- 2% (n = 10) of that at -100 mV. Based on these data, we hypothesized that voltage might alter the gating behavior of human CFTR. Using linear three-state kinetic schemes, we demonstrated that voltage has marked effects on channel gating. Membrane depolarization decreased both the duration of bursts and the interburst interval, but increased the duration of gaps within bursts. However, because the voltage dependencies of the different rate constants were in opposite directions, voltage was without large effect on the open probability (Po) of human CFTR. In contrast, the Po of murine CFTR was decreased markedly at positive voltages, suggesting that the rectification of murine CFTR is stronger than that of human CFTR. We conclude that inward rectification of CFTR is caused by a reduction in i and changes in gating kinetics. We suggest that inward rectification is an intrinsic property of the CFTR Cl- channel and not the result of pore block.
Figures


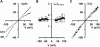


References
-
- Anderson, M.P., R.J. Gregory, S. Thompson, D.W. Souza, S. Paul, R.C. Mulligan, A.E. Smith, and M.J. Welsh. 1991. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 253:202–205. - PubMed
-
- Cai, Z., and D.N. Sheppard. 2002. Phloxine B interacts with the cystic fibrosis transmembrane conductance regulator at multiple sites to modulate channel activity. J. Biol. Chem. 277:19546–19553. - PubMed
-
- Carson, M.R., S.M. Travis, and M.J. Welsh. 1995. a. The two nucleotide-binding domains of cystic fibrosis transmembrane conductance regulator (CFTR) have distinct functions in controlling channel activity. J. Biol. Chem. 270:1711–1717. - PubMed
-
- Carson, M.R., M.C. Winter, S.M. Travis, and M.J. Welsh. 1995. b. Pyrophosphate stimulates wild-type and mutant cystic fibrosis transmembrane conductance regulator Cl− channels. J. Biol. Chem. 270:20466–20472. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

