Plasticity of repetitive DNA sequences within a bacterial (Type IV) secretion system component
- PMID: 14581606
- PMCID: PMC2194252
- DOI: 10.1084/jem.20030381
Plasticity of repetitive DNA sequences within a bacterial (Type IV) secretion system component
Abstract
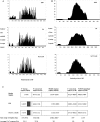
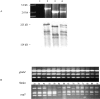
DNA rearrangement permits bacteria to regulate gene content and expression. In Helicobacter pylori, cagY, which contains an extraordinary number of direct DNA repeats, encodes a surface-exposed subunit of a (type IV) bacterial secretory system. Examining potential DNA rearrangements involving the cagY repeats indicated that recombination events invariably yield in-frame open reading frames, producing alternatively expressed genes. In individual hosts, H. pylori cell populations include strains that produce CagY proteins that differ in size, due to the predicted in-frame deletions or duplications, and elicit minimal or no host antibody recognition. Using repetitive DNA, H. pylori rearrangements in a host-exposed subunit of a conserved bacterial secretion system may permit a novel form of antigenic evasion.
Figures



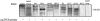

References
-
- Woolhouse, M.E., L.H. Taylor, and D.T. Haydon. 2001. Population biology of multihost pathogens. Science. 292:1109–1112. - PubMed
-
- Krinos, C.M., M.J. Coyne, K.G. Weinacht, A.O. Tzianabos, D.L. Kasper, and L.E. Comstock. 2001. Extensive surface diversity of a commensal microorganism by multiple DNA inversions. Nature. 414:555–558. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

