CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool
- PMID: 14581610
- PMCID: PMC2194245
- DOI: 10.1084/jem.20030916
CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool
Abstract
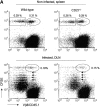
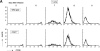
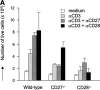
CD27, like CD28, acts in concert with the T cell receptor to support T cell expansion. Using CD27(-/-) mice, we have shown earlier that CD27 determines the magnitude of primary and memory T cell responses to influenza virus. Here, we have examined the relative contributions of CD27 and CD28 to generation of the virus-specific effector T cell pool and its establishment at the site of infection (the lung), using CD27(-/-), CD28(-/-), and CD27/CD28(-/-) mice. We find that primary and memory CD8+ T cell responses to influenza virus are dependent on the collective contribution of both receptors. In the primary response, CD27 and CD28 impact to a similar extent on expansion of virus-specific T cells in draining lymph nodes. CD27 is the principle determinant for accumulation of virus-specific T cells in the lung because it can sustain this response in CD28(-/-) mice. Unlike CD28, CD27 does not affect cell cycle activity, but promotes survival of activated T cells throughout successive rounds of division at the site of priming and may do so at the site of infection as well. CD27 was found to rescue CD28(-/-) T cells from death at the onset of division, explaining its capacity to support a T cell response in absence of CD28.
Figures




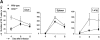
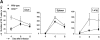




Similar articles
-
CD27 is required for generation and long-term maintenance of T cell immunity.Nat Immunol. 2000 Nov;1(5):433-40. doi: 10.1038/80877. Nat Immunol. 2000. PMID: 11062504
-
CD8+ T cells produce the chemokine CXCL10 in response to CD27/CD70 costimulation to promote generation of the CD8+ effector T cell pool.J Immunol. 2013 Sep 15;191(6):3025-36. doi: 10.4049/jimmunol.1202222. Epub 2013 Aug 12. J Immunol. 2013. PMID: 23940275
-
CD28 and CD27 costimulation of CD8+ T cells: a story of survival.Adv Exp Med Biol. 2007;590:149-70. doi: 10.1007/978-0-387-34814-8_11. Adv Exp Med Biol. 2007. PMID: 17191384 Review.
-
The Pim kinase pathway contributes to survival signaling in primed CD8+ T cells upon CD27 costimulation.J Immunol. 2010 Dec 1;185(11):6670-8. doi: 10.4049/jimmunol.1000159. Epub 2010 Nov 3. J Immunol. 2010. PMID: 21048108
-
Dendritic cells and CD28 costimulation are required to sustain virus-specific CD8+ T cell responses during the effector phase in vivo.J Immunol. 2011 Apr 15;186(8):4599-608. doi: 10.4049/jimmunol.1001972. Epub 2011 Mar 9. J Immunol. 2011. PMID: 21389258
Cited by
-
Co-Stimulatory Receptor Signaling in CAR-T Cells.Biomolecules. 2022 Sep 15;12(9):1303. doi: 10.3390/biom12091303. Biomolecules. 2022. PMID: 36139142 Free PMC article. Review.
-
The impact of a boosting immunogen on the differentiation of secondary memory CD8+ T cells.J Virol. 2007 Dec;81(23):12793-802. doi: 10.1128/JVI.01519-07. Epub 2007 Sep 19. J Virol. 2007. PMID: 17881444 Free PMC article.
-
Evaluation of the Effect of CD70 Co-Expression on CD8 T Cell Response in Protein-Prime MVA-Boost Vaccination in Mice.Vaccines (Basel). 2023 Jan 21;11(2):245. doi: 10.3390/vaccines11020245. Vaccines (Basel). 2023. PMID: 36851121 Free PMC article.
-
CD27 stimulation unveils the efficacy of linked class I/II peptide vaccines in poorly immunogenic tumors by orchestrating a coordinated CD4/CD8 T cell response.Oncoimmunology. 2018 Sep 5;7(12):e1502904. doi: 10.1080/2162402X.2018.1502904. eCollection 2018. Oncoimmunology. 2018. PMID: 30524899 Free PMC article.
-
CD1C is associated with breast cancer prognosis and immune infiltrates.BMC Cancer. 2023 Feb 8;23(1):129. doi: 10.1186/s12885-023-10558-2. BMC Cancer. 2023. PMID: 36755259 Free PMC article.
References
-
- Viola, A., S. Schroeder, Y. Sakakibara, and A. Lanzavecchia. 1999. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 283:680–682. - PubMed
-
- Noel, P.J., L.H. Boise, J.M. Green, and C.B. Thompson. 1996. CD28 costimulation prevents cell death during primary T cell activation. J. Immunol. 157:636–642. - PubMed
-
- Okkenhaug, K., L. Wu, K.M. Garza, J. La Rose, W. Khoo, N. Odermatt, T.W. Mak, P. Ohashi, and R. Rottapel. 2001. A point mutation in CD28 distinghuishes proliferative signals from survival signals. Nat. Immunol. 2:325–332. - PubMed
-
- Watts, T.H., and M.A. DeBenedette. 1999. T cell costimulatory molecules other than CD28. Curr. Opin. Immunol. 11:286–293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

