Abnormal osmotic regulation in trpv4-/- mice
- PMID: 14581612
- PMCID: PMC263876
- DOI: 10.1073/pnas.1735416100
Abnormal osmotic regulation in trpv4-/- mice
Abstract
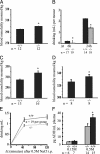
Osmotic homeostasis is one of the most aggressively defended physiological parameters in vertebrates. However, the molecular mechanisms underlying osmotic regulation are poorly understood. The transient receptor potential channel, vanilloid subfamily (TRPV4), is an osmotically activated ion channel that is expressed in circumventricular organs in the mammalian CNS, which is an important site of osmotic sensing. We have generated trpv4-null mice and observed abnormalities of their osmotic regulation. trpv4-/- mice drank less water and became more hyperosmolar than did wild-type littermates, a finding that was seen with and without administration of hypertonic saline. In addition, plasma levels of antidiuretic hormone were significantly lower in trpv4-/- mice than in wild-type littermates after a hyperosmotic challenge. Continuous s.c. infusion of the antidiuretic hormone analogue, dDAVP, resulted in systemic hypotonicity in trpv4-/- mice, despite the fact that their renal water reabsorption capacity was normal. Thus, the response to both hyper- and hypoosmolar stimuli is impaired in trpv4-/- mice. After a hyperosmolar challenge, there was markedly reduced expression of c-FOS in the circumventricular organ, the organum vasculosum of the lamina terminalis, of trpv4-/- mice compared with wild-type mice. This finding suggests that there is an impairment of osmotic sensing in the CNS of trpv4-/- mice. These data indicate that TRPV4 is necessary for the normal response to changes in osmotic pressure and functions as an osmotic sensor in the CNS.
Figures

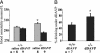

Similar articles
-
TRPV4 plays an evolutionary conserved role in the transduction of osmotic and mechanical stimuli in live animals.J Physiol. 2005 Aug 15;567(Pt 1):53-8. doi: 10.1113/jphysiol.2005.088963. Epub 2005 Jun 16. J Physiol. 2005. PMID: 15961428 Free PMC article. Review.
-
Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans.Proc Natl Acad Sci U S A. 2003 Nov 25;100 Suppl 2(Suppl 2):14531-6. doi: 10.1073/pnas.2235619100. Epub 2003 Oct 27. Proc Natl Acad Sci U S A. 2003. PMID: 14581619 Free PMC article.
-
When size matters: transient receptor potential vanilloid 4 channel as a volume-sensor rather than an osmo-sensor.J Physiol. 2017 Jun 1;595(11):3287-3302. doi: 10.1113/JP274135. Epub 2017 May 14. J Physiol. 2017. PMID: 28295351 Free PMC article.
-
Impaired osmotic sensation in mice lacking TRPV4.Am J Physiol Cell Physiol. 2003 Jul;285(1):C96-101. doi: 10.1152/ajpcell.00559.2002. Am J Physiol Cell Physiol. 2003. PMID: 12777254
-
Transient receptor potential vanilloid channels functioning in transduction of osmotic stimuli.J Endocrinol. 2006 Dec;191(3):515-23. doi: 10.1677/joe.1.07000. J Endocrinol. 2006. PMID: 17170210 Review.
Cited by
-
TRPV4-Mediated Regulation of the Blood Brain Barrier Is Abolished During Inflammation.Front Cell Dev Biol. 2020 Aug 27;8:849. doi: 10.3389/fcell.2020.00849. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32974355 Free PMC article.
-
Hyperglycemia and Diabetes Downregulate the Functional Expression of TRPV4 Channels in Retinal Microvascular Endothelium.PLoS One. 2015 Jun 5;10(6):e0128359. doi: 10.1371/journal.pone.0128359. eCollection 2015. PLoS One. 2015. PMID: 26047504 Free PMC article.
-
Translocation of TRPV2 channel induced by focal administration of mechanical stress.Physiol Rep. 2015 Feb 12;3(2):e12296. doi: 10.14814/phy2.12296. Print 2015 Feb 1. Physiol Rep. 2015. PMID: 25677550 Free PMC article.
-
Peripheral Mechanobiology of Touch-Studies on Vertebrate Cutaneous Sensory Corpuscles.Int J Mol Sci. 2020 Aug 27;21(17):6221. doi: 10.3390/ijms21176221. Int J Mol Sci. 2020. PMID: 32867400 Free PMC article. Review.
-
Upregulation of osmo-mechanosensitive TRPV4 channel facilitates chronic hypoxia-induced myogenic tone and pulmonary hypertension.Am J Physiol Lung Cell Mol Physiol. 2012 Mar 15;302(6):L555-68. doi: 10.1152/ajplung.00005.2011. Epub 2011 Dec 29. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22207590 Free PMC article.
References
-
- Bourque, C. W. & Oliet, S. H. (1997) Annu. Rev. Physiol. 59, 601-619. - PubMed
-
- Oldfield, B. J., Badoer, E., Hards, D. K. & McKinley, M. J. (1994) Neuroscience 60, 255-262. - PubMed
-
- Verney, E. B. (1947) Proc. R. Soc. London 135, 25-26. - PubMed
-
- Fitts, D. A., Starbuck, E. M. & Ruhf, A. (2000) Am. J. Physiol. 279, R2277-R2286. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

