Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading
- PMID: 14581613
- PMCID: PMC263848
- DOI: 10.1073/pnas.1735526100
Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading
Abstract
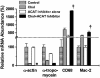
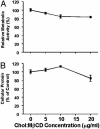
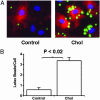
Mouse aortic smooth muscle cells (SMCs) were loaded for 72 h with cholesterol by using cholesterol:methyl-beta-cyclodextrin complexes, leading to approximately 2-fold and approximately 10-fold increases in the contents of total cholesterol and cholesteryl ester, respectively. Foam-cell formation was demonstrated by accumulation of intracellular, Oil Red O-stained lipid droplets. Immunostaining showed decreased protein levels of smooth muscle alpha-actin and alpha-tropomyosin and increased levels of macrophage markers CD68 and Mac-2 antigen. Quantitative real-time RT-PCR revealed that after cholesterol loading, the expression of SMC-related genes alpha-actin, alpha-tropomyosin, myosin heavy chain, and calponin H1 decreased (to 11.5 +/- 0.5%, 29.3 +/- 1.4%, 23.8 +/- 1.4%, and 3.8 +/- 0.5% of unloaded cells, respectively; P < 0.05 for all), whereas expression of macrophage-related genes CD68, Mac-2, and ABCA1 mRNA increased (to 709 +/- 84%, 330 +/- 11%, and 207 +/- 13% of unloaded cells, respectively; P < 0.05 for all), thereby demonstrating that the protein changes were regulated at the mRNA level. Furthermore, these changes were accompanied by a gain in macrophage-like function as assessed by phagocytotic activity. Expression of vascular cell adhesion molecule 1 and monocyte chemoattractant protein 1, known responders to inflammation, were not changed. In conclusion, cholesterol loading of SMC causes phenotypic changes regulated at the mRNA level that result in a transdifferentiation to a macrophage-like state. This finding suggests that not all foam cells in lesions may have a macrophage origin, despite what is indicated by immunostaining for macrophage-related markers. Furthermore, inflammatory changes in foam cells observed in vivo may not be simple consequences of cholesterol accumulation.
Figures


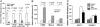
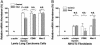
References
-
- Stary, H. C., Chandler, A. B., Glagov, S., Guyton, J. R., Insull, W., Jr., Rosenfeld, M. E., Schaffer, S. A., Schwartz, C. J., Wagner, W. D. & Wissler, R. W. (1994) Circulation 89, 2462-2478. - PubMed
-
- Boring, L., Gosling, J., Cleary, M. & Charo, I. F. (1998) Nature 394, 894-897. - PubMed
-
- Gu, L., Okada, Y., Clinton, S. K., Gerard, C., Sukhova, G. K., Libby, P. & Rollins, B. J. (1998) Mol. Cell 2, 275-281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

