The two rotor components of yeast mitochondrial ATP synthase are mechanically coupled by subunit delta
- PMID: 14581615
- PMCID: PMC263764
- DOI: 10.1073/pnas.2135169100
The two rotor components of yeast mitochondrial ATP synthase are mechanically coupled by subunit delta
Abstract
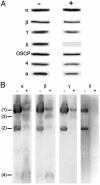
The mitochondrial ATP synthase is made of a membrane-integrated F0 component that forms a proton-permeable pore through the inner membrane and a globular peripheral F1 domain where ATP is synthesized. The catalytic mechanism is thought to involve the rotation of a 10-12 c subunit ring in the F0 together with the gamma subunit of F1. An important and not yet resolved question is to define precisely how the gamma subunit is connected with the c-ring. In this study, using a doxycycline-regulatable expression system, we provide direct evidence that the rest of the enzyme can assemble without the delta subunit of F1, and we show that delta-less mitochondria are uncoupled because of an F0-mediated proton leak. Based on these observations, and taking into account high-resolution structural models, we propose that subunit delta plays a key role in the mechanical coupling of the c-ring to subunit gamma.
Figures



References
-
- Boyer, P. D. (1997) Annu. Rev. Biochem. 66, 717-749. - PubMed
-
- Abrahams, J. P., Leslie, A. G. W., Lutter, R. & Walker, J. E. (1994) Nature 370, 621-628. - PubMed
-
- Gibbons, C., Montgomery, M. G., Leslie, A. G. W. & Walker, J. E. (2000) Nat. Struct. Biol. 7, 1055-1061. - PubMed
-
- Stock, D., Leslie, A. G. & Walker, J. E. (1999) Science 286, 1700-1705. - PubMed
-
- Fillingame, R. H. (2000) Nat. Struct. Biol. 7, 1002-1004. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

