Phase II trial of intralesional therapy with interleukin-2 in soft-tissue melanoma metastases
- PMID: 14583759
- PMCID: PMC2394422
- DOI: 10.1038/sj.bjc.6601320
Phase II trial of intralesional therapy with interleukin-2 in soft-tissue melanoma metastases
Abstract
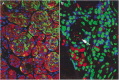
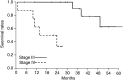
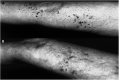
The objective of the present study was to validate the use of intralesional injection of interleukin-2 (IL-2) in patients with skin and soft-tissue melanoma metastases. A total of 24 patients with AJCC stage III or IV melanoma and single or multiple skin and soft-tissue metastases were included. Interleukin-2 injections were administered intralesionally into the total number of cutaneous and soft-tissue metastases accessible from the skin, 2-3 times weekly, over 1-57 weeks. Single doses varied from 0.6 to 6 x 10(6) IU, depending on lesion size. The clinical response was monitored by sonography and confirmed by histopathology; response evaluation was confined to the intralesionally treated tumours. Complete response (CR) of the treated metastases was achieved in 15 patients (62.5%), the longest remission lasting 38 months to date. In five patients, partial response (PR) was achieved (21%) and in another three patients, progressive disease was observed (one patient not assessable). A total of 245 metastases were treated with CR in 209 (85%), and PR in 21 (6%). The therapy was generally well tolerated; the observed adverse events were mainly of grade 1-2 severity. Immunohistochemical studies showed the tumour cells undergoing apoptosis and revealed a mixed character of the inflammatory infiltrate. The unusual high CR rate in metastatic melanoma of 62.5% and the limited toxicity suggest that treatment of skin and soft-tissue melanoma metastases with intralesional injection of IL-2 may be a safe and effective alternative to conventional therapies. The optimal dosage and duration of this therapy still remain to be defined in larger prospective multicentre trials.
Figures

References
-
- Abbas AK, Lichtman AH, Pober JS (1994) Effector mechanisms of T cell-mediated immune reactions; Immunity to tumors. In Cellular and molecular immunology, Abbas AK. (ed) pp 271–277, 357–375 Philadelphia: W. B. Saunders
-
- Burris HA, Vogel CL, Castro D, Mishra L, Schwarz M, Spencer S, Oakes DD, Korey A, Orenberg EK (1998) Intratumoral cisplatin/epinephrine-injectable gel as a palliative treatment for accessible solid tumors: a multicenter pilot study. Otolaryngol Head Neck Surg 118: 496–503 - PubMed
-
- Chikamatsu K, Reichert TE, Kashii Y, Saito T, Kawashiri S, Yamamoto E, Whiteside TL (1999) Immunotherapy with effector cells and IL-2 of lymph node metastases of human squamous-cell carcinoma of the head and neck established in nude mice. Int J Cancer 82: 532–537 - PubMed
-
- Cornejo P, Vanaclocha F, Polimon I, Del Rio R (2000) Intralesional interferon treatment of lentigo maligna. Arch Dermatol 136: 428–430 - PubMed
-
- Curiel-Lewandrowski C, Demierre MF (2000) Advances in specific immunotherapy of malignant melanoma. J Am Acad Dermatol 43: 167–188 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

