Ex vivo analysis of topotecan: advancing the application of laboratory-based clinical therapeutics
- PMID: 14583785
- PMCID: PMC2394409
- DOI: 10.1038/sj.bjc.6601336
Ex vivo analysis of topotecan: advancing the application of laboratory-based clinical therapeutics
Abstract
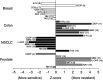
Topotecan is currently approved for relapsed small-cell lung cancer and ovarian cancer. Topotecan's efficacy in the second-line setting and novel mechanism of action suggest broad antitumour activity. We utilised a clinically validated, cell-death, ex vivo assay in human tumour explants to examine the activity profile of topotecan alone and in combination with other antitumour agents. Serial dilutions of topotecan alone and in combination with other cytotoxic agents were applied to biopsy specimens of non-small-cell lung cancer (NSCLC) and breast, colon, and prostate cancers. Dose-response curves were interpolated to provide 50% lethal concentrations (LC(50)). The degree of synergy (by median effect) and normalised Z-scores (raw scores converted to relative activity distributed around the mean) were then computed. Single-agent activity was observed for topotecan in all four tumour types. In 57 chemotherapy-naive specimens, NSCLC revealed the highest activity, demonstrated by the lowest LC(50) value (0.26+/-0.06 microg ml(-1); P=0.002). Overall, previously treated and chemotherapy-naive specimens revealed no significant differences in mean LC(50)'s. Synergy was observed for several combinations, including topotecan plus cisplatin in prostate and for topotecan plus 5-fluorouracil in breast cancers. The Z-score analyses conducted suggest activity for previously unexplored drug regimens, including topotecan plus 5-fluorouracil, vinorelbine, and mitomycin-C in NSCLC and breast cancer. Phase II studies are underway to determine the degree to which these ex vivo findings will translate into improved clinical results.
Figures


References
-
- Ardizzoni A, Hansen H, Dombernowsky P, Gamucci T, Kaplan S, Postmus P, Giaccone G, Schaefer B, Wanders J, Verweij J (1997) Topotecan, a new active drug in the second-line treatment of small-cell lung cancer: a phase II study in patients with refractory and sensitive disease. J Clin Oncol 15: 2090–2096 - PubMed
-
- Ardizzoni A, Manegold C, Gaafar R, Buchholdz E, Debruyne C, Damen S, Curran D, King K, Giaccone G (1999) Combination chemotherapy with cisplatin and topotecan as second-line treatment of sensitive (S) and refractory (R) small cell lung cancer (SCLC): an EORTC LCCG phase II study (abstract). Proc Am Soc Clin Oncol 18: 471a
-
- Beran M, Estey E, O'Brien SM, Giles FJ, Koller CA, Kornblau S, Keating M, Kantarjian HM (1998) Results of topotecan single-agent therapy in patients with myelodysplastic syndromes and chronic myelomonocytic leukemia. Leukemia Lymphoma 31: 521–531 - PubMed
-
- Bookman MA, Malmström H, Bolis G, Gordon A, Lissoni A, Krebs JB, Fields SZ (1998) Topotecan for the treatment of advanced epithelial ovarian cancer: an open-label phase II study in patients treated after prior chemotherapy that contained cisplatin or carboplatin and paclitaxel. J Clin Oncol 16: 3345–3352 - PubMed
-
- Bosanquet AG, Johnson SA, Richards SM (1999) Prognosis for fludarabine therapy of chronic lymphocytic leukaemia based on ex vivo drug response by DISC assay. Br J Haematol 106: 71–77 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

