ADP-ribosylation factor 6 regulates insulin secretion through plasma membrane phosphatidylinositol 4,5-bisphosphate
- PMID: 14585928
- PMCID: PMC263800
- DOI: 10.1073/pnas.2232129100
ADP-ribosylation factor 6 regulates insulin secretion through plasma membrane phosphatidylinositol 4,5-bisphosphate
Abstract
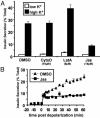
ADP-ribosylation factor 6 (ARF6) is a small GTP-binding protein that regulates peripheral vesicular trafficking and actin cytoskeletal dynamics, and it has been implicated as critical to regulated secretion. Expression of a dominant-inhibitory ARF6 mutant, ARF6(T27N), impaired glucose-, depolarization-, and gamma-thio-GTP-stimulated insulin secretion in the pancreatic beta cell line, MIN6. In response to depolarization, MIN6 cells expressing ARF6(T27N) displayed an unaltered initial fast phase but an impaired subsequent slow phase of insulin secretion. Actin cytoskeletal disassembly with latrunculin A enhanced insulin secretion, whereas stabilization with jasplakinolide inhibited secretion, consistent with the actin cytoskeleton serving as a barrier to exocytosis in these cells. ARF6(T27N) led to a depolarization-dependent reduction in the levels of phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] with a time course that paralleled the inhibition of secretion. Moreover, blockade of PI(4,5)P2-dependent events by expression of a lipid-binding protein resulted in inhibition of depolarization-induced secretion in a manner identical to ARF6(T27N). These results indicate that ARF6 is required to sustain adequate levels of PI(4,5)P2 during periods of increased PI(4,5)P2 metabolism such as regulated secretion.
Figures






Similar articles
-
G(alpha)11 signaling through ARF6 regulates F-actin mobilization and GLUT4 glucose transporter translocation to the plasma membrane.Mol Cell Biol. 2001 Aug;21(15):5262-75. doi: 10.1128/MCB.21.15.5262-5275.2001. Mol Cell Biol. 2001. PMID: 11438680 Free PMC article.
-
The GDP-bound form of Arf6 is located at the plasma membrane.J Cell Sci. 2004 May 1;117(Pt 11):2389-98. doi: 10.1242/jcs.01090. J Cell Sci. 2004. PMID: 15126638
-
Plasma Membrane Phosphatidylinositol 4,5-Bisphosphate Regulates Ca(2+)-Influx and Insulin Secretion from Pancreatic β Cells.Cell Chem Biol. 2016 Jul 21;23(7):816-826. doi: 10.1016/j.chembiol.2016.06.009. Cell Chem Biol. 2016. PMID: 27447049
-
Multiple roles for Arf6: sorting, structuring, and signaling at the plasma membrane.J Biol Chem. 2003 Oct 24;278(43):41573-6. doi: 10.1074/jbc.R300026200. Epub 2003 Aug 11. J Biol Chem. 2003. PMID: 12912991 Review. No abstract available.
-
Requirement for Arf6 in cell adhesion, migration, and cancer cell invasion.J Biochem. 2003 Oct;134(4):485-9. doi: 10.1093/jb/mvg181. J Biochem. 2003. PMID: 14607973 Review.
Cited by
-
Phospholipase D signaling: orchestration by PIP2 and small GTPases.Naunyn Schmiedebergs Arch Pharmacol. 2007 Feb;374(5-6):399-411. doi: 10.1007/s00210-007-0131-4. Epub 2007 Jan 24. Naunyn Schmiedebergs Arch Pharmacol. 2007. PMID: 17245604 Free PMC article. Review.
-
Phosphoinositide signalling in type 2 diabetes: a β-cell perspective.Biochem Soc Trans. 2016 Feb;44(1):293-8. doi: 10.1042/BST20150229. Biochem Soc Trans. 2016. PMID: 26862218 Free PMC article. Review.
-
YES, a Src family kinase, is a proximal glucose-specific activator of cell division cycle control protein 42 (Cdc42) in pancreatic islet β cells.J Biol Chem. 2014 Apr 18;289(16):11476-11487. doi: 10.1074/jbc.M114.559328. Epub 2014 Mar 7. J Biol Chem. 2014. PMID: 24610809 Free PMC article.
-
An integrative systems genetics approach reveals potential causal genes and pathways related to obesity.Genome Med. 2015 Oct 20;7:105. doi: 10.1186/s13073-015-0229-0. Genome Med. 2015. PMID: 26482556 Free PMC article.
-
Cellular and subcellular localization of ADP-ribosylation factor 6 in mouse peripheral tissues.Histochem Cell Biol. 2017 Dec;148(6):577-596. doi: 10.1007/s00418-017-1599-8. Epub 2017 Jul 26. Histochem Cell Biol. 2017. PMID: 28748255
References
-
- Donaldson, J. G. & Jackson, C. L. (2000) Curr. Opin. Cell Biol. 12, 475-482. - PubMed
-
- Moss, J. & Vaughan, M. (1998) J. Biol. Chem. 273, 21431-21434. - PubMed
-
- Caumont, A. S., Galas, M. C., Vitale, N., Aunis, D. & Bader, M. F. (1998) J. Biol. Chem. 273, 1373-1379. - PubMed
-
- Galas, M. C., Helms, J. B., Vitale, N., Thierse, D., Aunis, D. & Bader, M. F. (1997) J. Biol. Chem. 272, 2788-2793. - PubMed
-
- Caumont, A. S., Vitale, N., Gensse, M., Galas, M. C., Casanova, J. E. & Bader, M. F. (2000) J. Biol. Chem. 275, 15637-15644. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

