Adhesive and lateral E-cadherin dimers are mediated by the same interface
- PMID: 14585958
- PMCID: PMC262383
- DOI: 10.1128/MCB.23.22.7965-7972.2003
Adhesive and lateral E-cadherin dimers are mediated by the same interface
Abstract
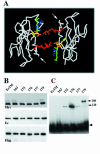
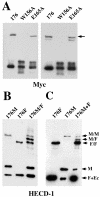
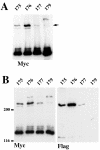
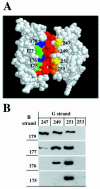
E-cadherin is a transmembrane protein that mediates Ca(2+)-dependent cell-cell adhesion. To study cadherin-cadherin interactions that may underlie the adhesive process, a recombinant E-cadherin lacking free sulfhydryl groups and its mutants with novel cysteines were expressed in epithelial A-431 cells. These cysteine mutants, designed according to various structural models of cadherin dimers, were constructed to reveal cadherin dimerization by the bifunctional sulfhydryl-specific cross-linker BM[PE0]3. Cross-linking experiments with the mutants containing a cysteine at strand B of their EC1 domains did show cadherin dimerization. By their properties these dimers correspond to those which have been characterized by co-immunoprecipitation assay. Under standard culture conditions the adhesive dimer is a dominant form. Calcium depletion dissociates adhesive dimers and promotes the formation of lateral dimers. Our data show that both dimers are mediated by the amino-terminal cadherin domain. Furthermore, the interfaces involved in both adhesive and lateral dimerization appear to be the same. The coexistence of the structurally identical adhesive and lateral dimers suggests some flexibility of the extracellular cadherin region.
Figures

Similar articles
-
Amino-terminal domain of classic cadherins determines the specificity of the adhesive interactions.J Cell Sci. 2000 Aug;113 ( Pt 16):2829-36. doi: 10.1242/jcs.113.16.2829. J Cell Sci. 2000. PMID: 10910767
-
Dynamic interplay between adhesive and lateral E-cadherin dimers.Mol Cell Biol. 2002 Nov;22(21):7449-58. doi: 10.1128/MCB.22.21.7449-7458.2002. Mol Cell Biol. 2002. PMID: 12370292 Free PMC article.
-
Sequential binding of calcium leads to dimerization in neural cadherin.Biochemistry. 2011 Apr 12;50(14):2973-82. doi: 10.1021/bi101872b. Epub 2011 Mar 21. Biochemistry. 2011. PMID: 21366346
-
Cadherin-mediated cell-cell adhesion: sticking together as a family.Curr Opin Struct Biol. 2003 Dec;13(6):690-8. doi: 10.1016/j.sbi.2003.10.007. Curr Opin Struct Biol. 2003. PMID: 14675546 Review.
-
Thinking outside the cell: how cadherins drive adhesion.Trends Cell Biol. 2012 Jun;22(6):299-310. doi: 10.1016/j.tcb.2012.03.004. Epub 2012 May 1. Trends Cell Biol. 2012. PMID: 22555008 Free PMC article. Review.
Cited by
-
Tuning the kinetics of cadherin adhesion.J Invest Dermatol. 2013 Oct;133(10):2318-2323. doi: 10.1038/jid.2013.229. Epub 2013 Jun 27. J Invest Dermatol. 2013. PMID: 23812234 Free PMC article. Review.
-
Regulation of N-cadherin dynamics at neuronal contacts by ligand binding and cytoskeletal coupling.Mol Biol Cell. 2006 Feb;17(2):862-75. doi: 10.1091/mbc.e05-04-0335. Epub 2005 Nov 30. Mol Biol Cell. 2006. PMID: 16319177 Free PMC article.
-
Dimeric states of neural- and epithelial-cadherins are distinguished by the rate of disassembly.Biochemistry. 2011 Apr 12;50(14):2951-61. doi: 10.1021/bi2001246. Epub 2011 Mar 21. Biochemistry. 2011. PMID: 21375242 Free PMC article.
-
Cadherins and catenins in dendrite and synapse morphogenesis.Cell Adh Migr. 2015;9(3):202-13. doi: 10.4161/19336918.2014.994919. Cell Adh Migr. 2015. PMID: 25914083 Free PMC article. Review.
-
Exploring the Nature of Desmosomal Cadherin Associations in 3D.Dermatol Res Pract. 2010;2010:930401. doi: 10.1155/2010/930401. Epub 2010 Jun 21. Dermatol Res Pract. 2010. PMID: 20672011 Free PMC article.
References
-
- Bibert, S., M. Jaquinod, E. Concord, C. Ebel, E. Hewat, C. Vanbelle, P. Legrand, M. Weidenhaupt, T. Vernet, and D. Gulino-Debrac. 2002. Synergy between extracellular modules of vascular endothelial cadherin promotes homotypic hexameric interactions. J. Biol. Chem. 277:12790-12801. - PubMed
-
- Blaschuk, O. W., and T. M. Rowlands. 2002. Plasma membrane components of adherens junctions. Mol. Membr. Biol. 19:75-80. - PubMed
-
- Boggon, T. J., J. Murray, S. Chappuis-Flament, E. Wong, B. M. Gumbiner, and L. Shapiro. 2002. C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science 296:1308-1313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
