Pyk2- and Src-dependent tyrosine phosphorylation of PDK1 regulates focal adhesions
- PMID: 14585963
- PMCID: PMC262336
- DOI: 10.1128/MCB.23.22.8019-8029.2003
Pyk2- and Src-dependent tyrosine phosphorylation of PDK1 regulates focal adhesions
Abstract
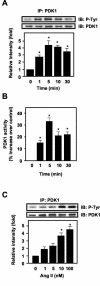
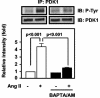
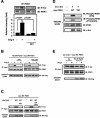
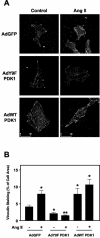
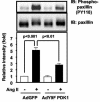
3-Phosphoinositide-dependent protein kinase 1 (PDK1) is a signal integrator that activates the AGC superfamily of serine/threonine kinases. PDK1 is phosphorylated on tyrosine by oxidants, although its regulation by agonists that stimulate G-protein-coupled receptor signaling pathways and the physiological consequences of tyrosine phosphorylation in this setting have not been fully identified. We found that angiotensin II stimulates the tyrosine phosphorylation of PDK1 in vascular smooth muscle in a calcium- and c-Src-dependent manner. The calcium-activated tyrosine kinase Pyk2 acts as a scaffold for Src-dependent phosphorylation of PDK1 on Tyr9, which permits phosphorylation of Tyr373 and -376 by Src. This critical function of Pyk2 is further supported by the observation that Pyk2 and tyrosine-phosphorylated PDK1 colocalize in focal adhesions after angiotensin II stimulation. Importantly, infection of smooth muscle cells with a Tyr9 mutant of PDK1 inhibits angiotensin II-induced tyrosine phosphorylation of paxillin and focal adhesion formation. These observations identify a novel interaction between PDK1 and Pyk2 that regulates the integrity of focal adhesions, which are major compartments for integrating signals for cell growth, apoptosis, and migration.
Figures



Similar articles
-
Calcium- and protein kinase C-dependent activation of the tyrosine kinase PYK2 by angiotensin II in vascular smooth muscle.Circ Res. 1998 Oct 19;83(8):841-51. doi: 10.1161/01.res.83.8.841. Circ Res. 1998. PMID: 9776731
-
A role for PYK2 in regulation of ERK1/2 MAP kinases and PI 3-kinase by ANG II in vascular smooth muscle.Am J Physiol Cell Physiol. 2001 Jan;280(1):C90-9. doi: 10.1152/ajpcell.2001.280.1.C90. Am J Physiol Cell Physiol. 2001. PMID: 11121380
-
Losartan improves aortic endothelium-dependent relaxation via proline-rich tyrosine kinase 2/Src/Akt pathway in type 2 diabetic Goto-Kakizaki rats.Am J Physiol Heart Circ Physiol. 2011 Dec;301(6):H2383-94. doi: 10.1152/ajpheart.00178.2011. Epub 2011 Sep 16. Am J Physiol Heart Circ Physiol. 2011. PMID: 21926342
-
Angiotensin II signal transduction in vascular smooth muscle: role of tyrosine kinases.Circ Res. 1997 May;80(5):607-16. doi: 10.1161/01.res.80.5.607. Circ Res. 1997. PMID: 9130441 Review.
-
Targeting vascular cell migration as a strategy for blocking angiogenesis: the central role of focal adhesion protein tyrosine kinase family.Curr Pharm Des. 2007;13(21):2129-45. doi: 10.2174/138161207781039643. Curr Pharm Des. 2007. PMID: 17627545 Review.
Cited by
-
Angiotensin II-induced aortic ring constriction is mediated by phosphatidylinositol 3-kinase/L-type calcium channel signaling pathway.Exp Mol Med. 2009 Aug 31;41(8):569-76. doi: 10.3858/emm.2009.41.8.062. Exp Mol Med. 2009. PMID: 19381068 Free PMC article.
-
TUSC4/NPRL2, a novel PDK1-interacting protein, inhibits PDK1 tyrosine phosphorylation and its downstream signaling.Cancer Sci. 2008 Sep;99(9):1827-34. doi: 10.1111/j.1349-7006.2008.00874.x. Epub 2008 Jul 4. Cancer Sci. 2008. PMID: 18616680 Free PMC article.
-
Evidence for contribution of common genetic variants within chromosome 8p21.2-8p21.1 to restricted and repetitive behaviors in autism spectrum disorders.BMC Genomics. 2016 Mar 1;17:163. doi: 10.1186/s12864-016-2475-y. BMC Genomics. 2016. PMID: 26931105 Free PMC article.
-
Arsenic alters vascular smooth muscle cell focal adhesion complexes leading to activation of FAK-src mediated pathways.Toxicol Appl Pharmacol. 2008 Sep 1;231(2):135-41. doi: 10.1016/j.taap.2008.04.002. Epub 2008 Apr 11. Toxicol Appl Pharmacol. 2008. PMID: 18486177 Free PMC article.
-
Slingshot isoform-specific regulation of cofilin-mediated vascular smooth muscle cell migration and neointima formation.Arterioscler Thromb Vasc Biol. 2011 Nov;31(11):2424-31. doi: 10.1161/ATVBAHA.111.232769. Arterioscler Thromb Vasc Biol. 2011. PMID: 21868701 Free PMC article.
References
-
- Alessi, D. R., M. Deak, A. Casamayor, F. B. Caudwell, N. Morrice, D. G. Norman, P. Gaffney, C. B. Reese, C. N. MacDougall, D. Harbison, A. Ashworth, and M. Bownes. 1997. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr. Biol. 7:776-789. - PubMed
-
- Alessi, D. R., S. R. James, C. P. Downes, A. B. Holmes, P. R. Gaffney, C. B. Reese, and P. Cohen. 1997. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase B alpha. Curr. Biol. 7:261-269. - PubMed
-
- Anderson, K. E., J. Coadwell, L. R. Stephens, and P. T. Hawkins. 1998. Translocation of PDK-1 to the plasma membrane is important in allowing PDK-1 to activate protein kinase B. Curr. Biol. 8:684-691. - PubMed
-
- Avraham, H., S. Y. Park, K. Schinkmann, and S. Avraham. 2000. RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 12:123-133. - PubMed
-
- Berk, B. C. 1999. Angiotensin II signal transduction in vascular smooth muscle: pathways activated by specific tyrosine kinases. J. Am. Soc. Nephrol. 10(Suppl. 11):S62-S68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
