Sox3 is required for gonadal function, but not sex determination, in males and females
- PMID: 14585968
- PMCID: PMC262333
- DOI: 10.1128/MCB.23.22.8084-8091.2003
Sox3 is required for gonadal function, but not sex determination, in males and females
Abstract
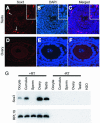
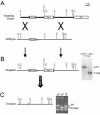
Sox3 is expressed in developing gonads and in the brain. Evolutionary evidence suggests that the X-chromosomal Sox3 gene may be the ancestral precursor of Sry, a sex-determining gene, and Sox3 has been proposed to play a role in sex determination. However, patients with mutations in SOX3 exhibit normal gonadal determination but are mentally retarded and have short stature secondary to growth hormone (GH) deficiency. We used Cre-LoxP targeted mutagenesis to delete Sox3 from mice. Null mice of both sexes had no overt behavioral deficits and exhibited normal GH gene expression. Low body weight was observed for some mice; overgrowth and misalignment of the front teeth was observed consistently. Female Sox3 null mice (-/-) developed ovaries but had excess follicular atresia, ovulation of defective oocytes, and severely reduced fertility. Pituitary (luteinizing hormone and follicle-stimulating hormone) and uterine functions were normal in females. Hemizygous male null mice (-/Y) developed testes but were hypogonadal. Testis weight was reduced by 42%, and there was extensive Sertoli cell vacuolization, loss of germ cells, reduced sperm counts, and disruption of the seminiferous tubules. We conclude that Sox3 is not required for gonadal determination but is important for normal oocyte development and male testis differentiation and gametogenesis.
Figures



Similar articles
-
Sox3 expression in undifferentiated spermatogonia is required for the progression of spermatogenesis.Dev Biol. 2005 Jul 1;283(1):215-25. doi: 10.1016/j.ydbio.2005.04.013. Dev Biol. 2005. PMID: 15893302
-
Differential expression and dynamic changes of SOX3 during gametogenesis and sex reversal in protogynous hermaphroditic fish.J Exp Zool A Ecol Genet Physiol. 2007 Apr 1;307(4):207-19. doi: 10.1002/jez.361. J Exp Zool A Ecol Genet Physiol. 2007. PMID: 17436330
-
Mutation analysis of subjects with 46, XX sex reversal and 46, XY gonadal dysgenesis does not support the involvement of SOX3 in testis determination.Hum Genet. 2000 Dec;107(6):650-2. doi: 10.1007/s004390000428. Epub 2000 Nov 14. Hum Genet. 2000. PMID: 11153920
-
From brain determination to testis determination: evolution of the mammalian sex-determining gene.Reprod Fertil Dev. 2001;13(7-8):665-72. doi: 10.1071/rd01093. Reprod Fertil Dev. 2001. PMID: 11999319 Review.
-
Interactions between SRY and SOX genes in mammalian sex determination.Bioessays. 1998 Mar;20(3):264-9. doi: 10.1002/(SICI)1521-1878(199803)20:3<264::AID-BIES10>3.0.CO;2-1. Bioessays. 1998. PMID: 9631654 Review.
Cited by
-
Array comparative genomic hybridisation analysis of boys with X linked hypopituitarism identifies a 3.9 Mb duplicated critical region at Xq27 containing SOX3.J Med Genet. 2004 Sep;41(9):669-78. doi: 10.1136/jmg.2003.016949. J Med Genet. 2004. PMID: 15342697 Free PMC article.
-
SOX2 is a dose-dependent regulator of retinal neural progenitor competence.Genes Dev. 2006 May 1;20(9):1187-202. doi: 10.1101/gad.1407906. Genes Dev. 2006. PMID: 16651659 Free PMC article.
-
Similarities and differences of X and Y chromosome homologous genes, SRY and SOX3, in regulating the renin-angiotensin system promoters.Physiol Genomics. 2015 May;47(5):177-86. doi: 10.1152/physiolgenomics.00138.2014. Epub 2015 Mar 10. Physiol Genomics. 2015. PMID: 25759379 Free PMC article.
-
Translational genetics for diagnosis of human disorders of sex development.Annu Rev Genomics Hum Genet. 2013;14:371-92. doi: 10.1146/annurev-genom-091212-153417. Epub 2013 Jul 15. Annu Rev Genomics Hum Genet. 2013. PMID: 23875799 Free PMC article. Review.
-
The mammalian ovary from genesis to revelation.Endocr Rev. 2009 Oct;30(6):624-712. doi: 10.1210/er.2009-0012. Epub 2009 Sep 23. Endocr Rev. 2009. PMID: 19776209 Free PMC article. Review.
References
-
- Albrecht, K. H., and E. M. Eicher. 2001. Evidence that Sry is expressed in pre-Sertoli cells and Sertoli and granulosa cells have a common precursor. Dev. Biol. 240:92-107. - PubMed
-
- Anawalt, B. D., R. A. Bebb, A. M. Matsumoto, N. P. Groome, P. J. Illingworth, A. S. McNeilly, and W. J. Bremner. 1996. Serum inhibin B levels reflect Sertoli cell function in normal men and men with testicular dysfunction. J. Clin. Endocrinol. Metab. 81:3341-3345. - PubMed
-
- Anderson, R. A., and R. M. Sharpe. 2000. Regulation of inhibin production in the human male and its clinical applications. Int. J. Androl. 23:136-144. - PubMed
-
- Bartles, J. R., A. Wierda, and L. Zheng. 1996. Identification and characterization of espin, an actin-binding protein localized to the F-actin-rich junctional plaques of Sertoli cell ectoplasmic specializations. J. Cell Sci. 109:1229-1239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
