Biochemical characterization of the Ran-RanBP1-RanGAP system: are RanBP proteins and the acidic tail of RanGAP required for the Ran-RanGAP GTPase reaction?
- PMID: 14585972
- PMCID: PMC262373
- DOI: 10.1128/MCB.23.22.8124-8136.2003
Biochemical characterization of the Ran-RanBP1-RanGAP system: are RanBP proteins and the acidic tail of RanGAP required for the Ran-RanGAP GTPase reaction?
Abstract
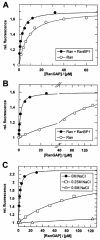
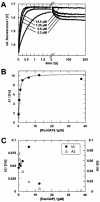
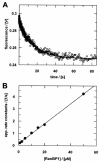
RanBP type proteins have been reported to increase the catalytic efficiency of the RanGAP-mediated GTPase reaction on Ran. Since the structure of the Ran-RanBP1-RanGAP complex showed RanBP1 to be located away from the active site, we reinvestigated the reaction using fluorescence spectroscopy under pre-steady-state conditions. We can show that RanBP1 indeed does not influence the rate-limiting step of the reaction, which is the cleavage of GTP and/or the release of product P(i). It does, however, influence the dynamics of the Ran-RanGAP interaction, its most dramatic effect being the 20-fold stimulation of the already very fast association reaction such that it is under diffusion control (4.5 x 10(8) M(-1) s(-1)). Having established a valuable kinetic system for the interaction analysis, we also found, in contrast to previous findings, that the highly conserved acidic C-terminal end of RanGAP is not required for the switch-off reaction. Rather, genetic experiments in Saccharomyces cerevisiae demonstrate a profound effect of the acidic tail on microtubule organization during mitosis. We propose that the acidic tail of RanGAP is required for a process during mitosis.
Figures











Similar articles
-
Random mutagenesis and functional analysis of the Ran-binding protein, RanBP1.J Biol Chem. 2000 Feb 11;275(6):4081-91. doi: 10.1074/jbc.275.6.4081. J Biol Chem. 2000. PMID: 10660567
-
The acidic C-terminal domain of rna1p is required for the binding of Ran.GTP and for RanGAP activity.J Biol Chem. 1997 Sep 26;272(39):24717-26. doi: 10.1074/jbc.272.39.24717. J Biol Chem. 1997. PMID: 9305944
-
Ran-binding protein 1 (RanBP1) forms a ternary complex with Ran and karyopherin beta and reduces Ran GTPase-activating protein (RanGAP) inhibition by karyopherin beta.J Biol Chem. 1997 Jan 3;272(1):551-5. doi: 10.1074/jbc.272.1.551. J Biol Chem. 1997. PMID: 8995296
-
RCC1 in the Ran pathway.J Biochem. 1996 Aug;120(2):207-14. doi: 10.1093/oxfordjournals.jbchem.a021400. J Biochem. 1996. PMID: 8889801 Review.
-
Physical breakdown of the nuclear envelope is not necessary for breaking its barrier function.Nucleus. 2011 Nov-Dec;2(6):523-6. doi: 10.4161/nucl.2.6.16117. Epub 2011 Nov 1. Nucleus. 2011. PMID: 22064471 Free PMC article. Review.
Cited by
-
Small GTP-binding protein Ran is regulated by posttranslational lysine acetylation.Proc Natl Acad Sci U S A. 2015 Jul 14;112(28):E3679-88. doi: 10.1073/pnas.1505995112. Epub 2015 Jun 29. Proc Natl Acad Sci U S A. 2015. PMID: 26124124 Free PMC article.
-
Interactions between secreted GRA proteins and host cell proteins across the paratitophorous vacuolar membrane in the parasitism of Toxoplasma gondii.Korean J Parasitol. 2006 Dec;44(4):303-12. doi: 10.3347/kjp.2006.44.4.303. Korean J Parasitol. 2006. PMID: 17170572 Free PMC article.
-
Localized regulation of axonal RanGTPase controls retrograde injury signaling in peripheral nerve.Neuron. 2008 Jul 31;59(2):241-52. doi: 10.1016/j.neuron.2008.05.029. Neuron. 2008. PMID: 18667152 Free PMC article.
-
Factor quinolinone inhibitors disrupt spindles and multiple LSF (TFCP2)-protein interactions in mitosis, including with microtubule-associated proteins.PLoS One. 2022 Jun 15;17(6):e0268857. doi: 10.1371/journal.pone.0268857. eCollection 2022. PLoS One. 2022. PMID: 35704642 Free PMC article.
-
B-type Plexins promote the GTPase activity of Ran to affect androgen receptor nuclear translocation in prostate cancer.Cancer Gene Ther. 2023 Nov;30(11):1513-1523. doi: 10.1038/s41417-023-00655-6. Epub 2023 Aug 10. Cancer Gene Ther. 2023. PMID: 37563360 Free PMC article.
References
-
- Agatep, R., R. D. Kirkpatrick, D. L. Parchaliuk, R. A. Woods, and R. D. Gietz. 1998. Transformation of Saccharomyces cerevisiae by the lithium acetate/single-stranded carrier DNA/polyethylene glycol (LiAc/ss-DNA/PEG) protocol. Tech. Tips Online 1:P01525. [Online.] http://research.bmn.com/tto.
-
- Ahmadian, M. R., U. Hoffmann, R. S. Goody, and A. Wittinghofer. 1997. Individual rate constants for the interaction of Ras proteins with GTPase-activating proteins determined by fluorescence spectroscopy. Biochemistry 36:4535-4541. - PubMed
-
- Ahmadian, M. R., R. Mittal, A. Hall, and A. Wittinghofer. 1997. Aluminum fluoride associates with the small guanine nucleotide binding proteins. FEBS Lett. 408:315-318. - PubMed
-
- Ahmadian, M. R., P. Stege, K. Scheffzek, and A. Wittinghofer. 1997. Confirmation of the arginine-finger hypothesis for the GAP-stimulated GTP-hydrolysis reaction of Ras. Nat. Struct. Biol. 4:686-689. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
