The Ku heterodimer performs separable activities at double-strand breaks and chromosome termini
- PMID: 14585978
- PMCID: PMC262345
- DOI: 10.1128/MCB.23.22.8202-8215.2003
The Ku heterodimer performs separable activities at double-strand breaks and chromosome termini
Abstract
The Ku heterodimer functions at two kinds of DNA ends: telomeres and double-strand breaks. The role that Ku plays at these two classes of termini must be distinct, because Ku is required for accurate and efficient joining of double-strand breaks while similar DNA repair events are normally prohibited at chromosome ends. Toward defining these functional differences, we have identified eight mutations in the large subunit of the Saccharomyces cerevisiae Ku heterodimer (YKU80) which retain the ability to repair double-strand breaks but are severely impaired for chromosome end protection. Detailed characterization of these mutations, referred to as yku80(tel) alleles, has revealed that Ku performs functionally distinct activities at subtelomeric chromatin versus the end of the chromosome, and these activities are separable from Ku's role in telomere length regulation. While at the chromosome terminus, we propose that Ku participates in two different activities: it facilitates telomerase-mediated G-strand synthesis, thereby contributing to telomere length regulation, and it separately protects against resection of the C-strand, thereby contributing to protection of chromosome termini. Furthermore, we propose that the Ku heterodimer performs discrete sets of functions at chromosome termini and at duplex subtelomeric chromatin, via separate interactions with these two locations. Based on homology modeling with the human Ku structure, five of the yku80(tel) alleles mutate residues that are conserved between the yeast and human Ku80 proteins, suggesting that these mutations probe activities that are shared between yeast and humans.
Figures
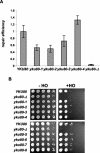






References
-
- Aparicio, O. M. 1999. Characterization of protein bound to chromatin by immunoprecipitation from whole-cell extracts, p. 21.3.1-21.3.12. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
-
- Aparicio, O. M., and D. E. Gottschling. 1994. Overcoming telomeric silencing: a trans-activator competes to establish gene expression in a cell cycle-dependent way. Genes Dev. 8:1133-1146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
