Neurons expressing the highest levels of gamma-synuclein are unaffected by targeted inactivation of the gene
- PMID: 14585981
- PMCID: PMC262405
- DOI: 10.1128/MCB.23.22.8233-8245.2003
Neurons expressing the highest levels of gamma-synuclein are unaffected by targeted inactivation of the gene
Abstract
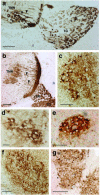
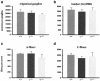
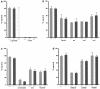
Homologous recombination in ES cells was employed to generate mice with targeted deletion of the first three exons of the gamma-synuclein gene. Complete inactivation of gene expression in null mutant mice was confirmed on the mRNA and protein levels. Null mutant mice are viable, are fertile, and do not display evident phenotypical abnormalities. The effects of gamma-synuclein deficiency on motor and peripheral sensory neurons were studied by various methods in vivo and in vitro. These two types of neurons were selected because they both express high levels of gamma-synuclein from the early stages of mouse embryonic development but later in the development they display different patterns of intracellular compartmentalization of the protein. We found no difference in the number of neurons between wild-type and null mutant animals in several brain stem motor nuclei, in lumbar dorsal root ganglia, and in the trigeminal ganglion. The survival of gamma-synuclein-deficient trigeminal neurons in various culture conditions was not different from that of wild-type neurons. There was no difference in the numbers of myelinated and nonmyelinated fibers in the saphenous nerves of these animals, and sensory reflex thresholds were also intact in gamma-synuclein null mutant mice. Nerve injury led to similar changes in sensory function in wild-type and mutant mice. Taken together, our data suggest that like alpha-synuclein, gamma-synuclein is dispensable for the development and function of the nervous system.
Figures





Similar articles
-
Peripheral sensory neurons survive in the absence of alpha- and gamma-synucleins.J Mol Neurosci. 2005;25(2):157-64. doi: 10.1385/JMN:25:2:157. J Mol Neurosci. 2005. PMID: 15784963
-
Developmental loss and resistance to MPTP toxicity of dopaminergic neurones in substantia nigra pars compacta of gamma-synuclein, alpha-synuclein and double alpha/gamma-synuclein null mutant mice.J Neurochem. 2004 Jun;89(5):1126-36. doi: 10.1111/j.1471-4159.2004.02378.x. J Neurochem. 2004. PMID: 15147505
-
Persyn, a member of the synuclein family, has a distinct pattern of expression in the developing nervous system.J Neurosci. 1998 Nov 15;18(22):9335-41. doi: 10.1523/JNEUROSCI.18-22-09335.1998. J Neurosci. 1998. PMID: 9801372 Free PMC article.
-
Localization of synucleins in the mammalian cochlea.J Assoc Res Otolaryngol. 2008 Dec;9(4):452-63. doi: 10.1007/s10162-008-0134-y. Epub 2008 Jul 30. J Assoc Res Otolaryngol. 2008. PMID: 18665422 Free PMC article.
-
Deletion of the alpha-synuclein locus in a subpopulation of C57BL/6J inbred mice.BMC Neurosci. 2001;2:11. doi: 10.1186/1471-2202-2-11. Epub 2001 Aug 24. BMC Neurosci. 2001. PMID: 11591219 Free PMC article.
Cited by
-
Gamma-synucleinopathy: neurodegeneration associated with overexpression of the mouse protein.Hum Mol Genet. 2009 May 15;18(10):1779-94. doi: 10.1093/hmg/ddp090. Epub 2009 Feb 26. Hum Mol Genet. 2009. PMID: 19246516 Free PMC article.
-
Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro.Science. 2010 Sep 24;329(5999):1663-7. doi: 10.1126/science.1195227. Epub 2010 Aug 26. Science. 2010. PMID: 20798282 Free PMC article.
-
Hindering of proteinopathy-induced neurodegeneration as a new mechanism of action for neuroprotectors and cognition enhancing compounds.Dokl Biochem Biophys. 2009 Sep-Oct;428:235-8. doi: 10.1134/s1607672909050032. Dokl Biochem Biophys. 2009. PMID: 20848907 No abstract available.
-
Targets of tyrosine nitration in diabetic rat retina.Mol Cell Proteomics. 2008 May;7(5):864-74. doi: 10.1074/mcp.M700417-MCP200. Epub 2007 Dec 28. Mol Cell Proteomics. 2008. Retraction in: Mol Cell Proteomics. 2011 Aug;10(8):A700417MCP200. doi: 10.1074/mcp.A700417-MCP200. PMID: 18165258 Free PMC article. Retracted.
-
Gene expression profiling of two distinct neuronal populations in the rodent spinal cord.PLoS One. 2008;3(10):e3415. doi: 10.1371/journal.pone.0003415. Epub 2008 Oct 15. PLoS One. 2008. PMID: 18923679 Free PMC article.
References
-
- Abeliovich, A., Y. Schmitz, I. Farinas, D. Choi-Lundberg, W. H. Ho, P. E. Castillo, N. Shinsky, J. M. Verdugo, M. Armanini, A. Ryan, M. Hynes, H. Phillips, D. Sulzer, and A. Rosenthal. 2000. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25:239-252. - PubMed
-
- Alimova-Kost, M. V., N. N. Ninkina, S. Imreh, N. V. Gnuchev, J. Adu, A. M. Davies, and V. L. Buchman. 1999. Genomic structure and chromosomal localization of the mouse persyn gene. Genomics 56:224-227. - PubMed
-
- Bennett, G. L., and Y.-K. Xie. 1988. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33:87-109. - PubMed
-
- Biere, A. L., S. J. Wood, J. Wypych, S. Steavenson, Y. Jiang, D. Anafi, F. W. Jacobsen, M. A. Jarosinski, G. M. Wu, J. C. Louis, F. Martin, L. O. Narhi, and M. Citron. 2000. Parkinson's disease-associated alpha-synuclein is more fibrillogenic than beta- and gamma-synuclein and cannot cross-seed its homologs. J. Biol. Chem. 275:34574-34579. - PubMed
-
- Blackburn-Munro, G., and S. M. Fleetwood-Walker. 1999. The sodium channel auxiliary subunits beta1 and beta2 are differentially expressed in the spinal cord of neuropathic rats. Neuroscience 90:153-164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
