Functional characterization of a novel promoter element required for an innate immune response in Drosophila
- PMID: 14585984
- PMCID: PMC262376
- DOI: 10.1128/MCB.23.22.8272-8281.2003
Functional characterization of a novel promoter element required for an innate immune response in Drosophila
Abstract
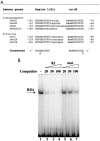
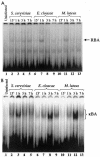
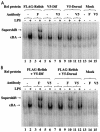
Innate immune reactions are crucial processes of metazoans to protect the organism against overgrowth of faster replicating microorganisms. Drosophila melanogaster is a precious model for genetic and molecular studies of the innate immune system. In response to infection, the concerted action of a battery of antimicrobial peptides ensures efficient killing of the microbes. The induced gene expression relies on translocation of the Drosophila Rel transcription factors Relish, Dif, and Dorsal to the nucleus where they bind to kappaB-like motifs in the promoters of the inducible genes. We have identified another putative promoter element, called region 1 (R1), in a number of antimicrobial peptide genes. Site-directed mutagenesis of the R1 site diminished Cecropin A1 (CecA1) expression in transgenic Drosophila larvae and flies. Infection of flies induced a nuclear R1-binding activity that was unrelated to the kappaB-binding activity in the same extracts. Although the R1 motif was required for Rel protein-mediated CecA1 expression in cotransfection experiments, our data argue against it being a direct target for the Drosophila Rel proteins. We propose that the R1 and kappaB motifs are targets for distinct regulatory complexes that act in concert to promote high levels of antimicrobial peptide gene expression in response to infection.
Figures



Similar articles
-
An in vitro study of NF-κB factors cooperatively in regulation of Drosophila melanogaster antimicrobial peptide genes.Dev Comp Immunol. 2019 Jun;95:50-58. doi: 10.1016/j.dci.2019.01.017. Epub 2019 Feb 6. Dev Comp Immunol. 2019. PMID: 30735676
-
Functional characterization of the infection-inducible peptide Edin in Drosophila melanogaster.PLoS One. 2012;7(5):e37153. doi: 10.1371/journal.pone.0037153. Epub 2012 May 14. PLoS One. 2012. PMID: 22606343 Free PMC article.
-
Serpent regulates Drosophila immunity genes in the larval fat body through an essential GATA motif.EMBO J. 1999 Jul 15;18(14):4013-22. doi: 10.1093/emboj/18.14.4013. EMBO J. 1999. PMID: 10406806 Free PMC article.
-
Antimicrobial peptides in Drosophila: structures, activities and gene regulation.Chem Immunol Allergy. 2005;86:1-21. doi: 10.1159/000086648. Chem Immunol Allergy. 2005. PMID: 15976485 Review.
-
Overview of Drosophila immunity: a historical perspective.Dev Comp Immunol. 2014 Jan;42(1):3-15. doi: 10.1016/j.dci.2013.08.018. Epub 2013 Sep 4. Dev Comp Immunol. 2014. PMID: 24012863 Review.
Cited by
-
Nubbin isoform antagonism governs Drosophila intestinal immune homeostasis.PLoS Pathog. 2018 Mar 2;14(3):e1006936. doi: 10.1371/journal.ppat.1006936. eCollection 2018 Mar. PLoS Pathog. 2018. PMID: 29499056 Free PMC article.
-
A novel association between clustered NF-kappaB and C/EBP binding sites is required for immune regulation of mosquito Defensin genes.Insect Mol Biol. 2006 Aug;15(4):393-401. doi: 10.1111/j.1365-2583.2006.00635.x. Insect Mol Biol. 2006. PMID: 16907826 Free PMC article.
-
Inhibitor kappaB-like proteins from a polydnavirus inhibit NF-kappaB activation and suppress the insect immune response.Proc Natl Acad Sci U S A. 2005 Aug 9;102(32):11426-31. doi: 10.1073/pnas.0505240102. Epub 2005 Aug 1. Proc Natl Acad Sci U S A. 2005. PMID: 16061795 Free PMC article.
-
NF-κB/Rel proteins and the humoral immune responses of Drosophila melanogaster.Curr Top Microbiol Immunol. 2011;349:25-60. doi: 10.1007/82_2010_107. Curr Top Microbiol Immunol. 2011. PMID: 20852987 Free PMC article. Review.
-
Dietary Curcumin Intake and Its Effects on the Transcriptome and Metabolome of Drosophila melanogaster.Int J Mol Sci. 2024 Jun 14;25(12):6559. doi: 10.3390/ijms25126559. Int J Mol Sci. 2024. PMID: 38928266 Free PMC article.
References
-
- Boman, H. G., I. Nilsson, and B. Rasmuson. 1972. Inducible antibacterial defence system in Drosophila. Nature 237:232-235. - PubMed
-
- Boutros, M., H. Agaisse, and N. Perrimon. 2002. Sequential activation of signaling pathways during innate immune responses in Drosophila. Dev. Cell 3:711-722. - PubMed
-
- Bulet, P., C. Hetru, J.-L. Dimarcq, and D. Hoffmann. 1999. Antimicrobial peptides in insects; structure and function. Dev. Comp. Immunol. 23:329-344. - PubMed
-
- Cantera, R., E. Roos, and Y. Engström. 1999. Dif and cactus are colocalized in the larval nervous system of Drosophila melanogaster. J. Neurobiol. 38:16-26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
