Different mechanisms promote astrocyte Ca2+ waves and spreading depression in the mouse neocortex
- PMID: 14586018
- PMCID: PMC6740882
- DOI: 10.1523/JNEUROSCI.23-30-09888.2003
Different mechanisms promote astrocyte Ca2+ waves and spreading depression in the mouse neocortex
Abstract
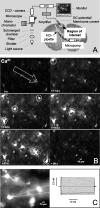
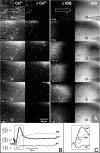
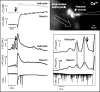
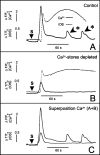
Cortical spreading depression (CSD) is thought to play an important role in different pathological conditions of the human brain. Here we investigated the interaction between CSD and Ca2+ waves within the astrocyte population in slices from mouse neocortex (postnatal days 10-14). After local KCl ejection as a trigger for CSD, we recorded the propagation of Ca2+ increases within a large population of identified astrocytes in synchrony with CSD measured as intrinsic optical signal (IOS) or negative DC-potential shift. The two events spread with 39.2 +/- 3.3 mum/sec until the IOS and negative DC-potential shift decayed after approximately 1 mm. However, the astrocyte Ca2+ wave continued to propagate for up to another 500 microm but with a reduced speed of 18.3 +/- 2.5 microm/sec that is also typical for glial Ca2+ waves in white matter or culture. While blocking CSD using MK-801 (40 microm), an NMDA-receptor antagonist, the astrocyte Ca2+ wave persisted with a reduced speed (13.2 +/- 1.5 microm/sec). The specific gap junction blocker carbenoxolon (100 microm) did not prevent CSD but decelerated the speed (2.9 +/- 0.9 microm/sec) of the astrocyte Ca2+ wave in the periphery of CSD. We also found that interfering with intracellular astrocytic Ca2+ signaling by depletion of internal Ca2+ stores does not affect the spread of the IOS. We conclude that CSD determines the velocity of an accompanying astrocytic Ca2+ response, but the astrocyte Ca2+ wave penetrates a larger territory and by this represents a self-reliant phenomenon with a different mechanism of propagation.
Figures




References
-
- Anderson TR, Andrew RD ( 2002) Spreading depression: imaging and blockade in the rat neocortical brain slice. J Neurophysiol 88: 2713–2725. - PubMed
-
- Araque A, Carmignoto G, Haydon PG ( 2001) Dynamic signaling between astrocytes and neurons. Annu Rev Physiol 63: 795–813. - PubMed
-
- Back T, Ginsberg MD, Dietrich WD, Watson BD ( 1996) Induction of spreading depression in the ischemic hemisphere following experimental middle cerebral artery occlusion: effect on infarct morphology. J Cereb Blood Flow Metab 16: 202–213. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
