Vacuolating encephalitis in mice infected by human coronavirus OC43
- PMID: 14592756
- PMCID: PMC7126296
- DOI: 10.1016/s0042-6822(03)00323-4
Vacuolating encephalitis in mice infected by human coronavirus OC43
Abstract
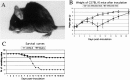
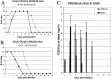
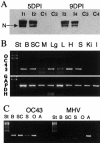
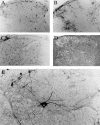
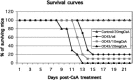
Involvement of viruses in human neurodegenerative diseases and the underlying pathologic mechanisms remain generally unclear. Human respiratory coronaviruses (HCoV) can infect neural cells, persist in human brain, and activate myelin-reactive T cells. As a means of understanding the human infection, we characterized in vivo the neurotropic and neuroinvasive properties of HCoV-OC43 through the development of an experimental animal model. Virus inoculation of 21-day postnatal C57BL/6 and BALB/c mice led to a generalized infection of the whole CNS, demonstrating HCoV-OC43 neuroinvasiveness and neurovirulence. This acute infection targeted neurons, which underwent vacuolation and degeneration while infected regions presented strong microglial reactivity and inflammatory reactions. Damage to the CNS was not immunologically mediated and microglial reactivity was instead a consequence of direct virus-mediated neuronal injury. Although this acute encephalitis appears generally similar to that induced by murine coronaviruses, an important difference rests in the prominent spongiform-like degeneration that could trigger neuropathology in surviving animals.
Figures



References
-
- Agapitos E., Pavlopoulos P.M., Patsouris E., Davaris P. Subacute necrotizing encephalopathy (Leigh's disease): a clinicopathologic study of ten cases. Gen. Diag. Pathol. 1997;142:341–355. - PubMed
-
- Allen I.V., McQuaid S., McMahon J., Kirk J., McConnell R. The significance of measles virus antigen and genome distribution in the CNS in SSPE for mechanisms of viral spread and demyelination. J. Neuropathol. Exp. Neurol. 1996;55:471–480. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

