PP1 control of M phase entry exerted through 14-3-3-regulated Cdc25 dephosphorylation
- PMID: 14592972
- PMCID: PMC275402
- DOI: 10.1093/emboj/cdg545
PP1 control of M phase entry exerted through 14-3-3-regulated Cdc25 dephosphorylation
Abstract
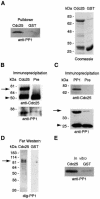
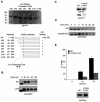
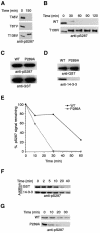
It has been known for over a decade that inhibition of protein phosphatase 1 (PP1) activity prevents entry into M phase, but the relevant substrate has not been identified. We report here that PP1 is required for dephosphorylation of the Cdc2-directed phosphatase Cdc25 at Ser287 (of Xenopus Cdc25; Ser216 of human Cdc25C), a site that suppresses Cdc25 during interphase. Moreover, PP1 recognizes Cdc25 directly by interacting with a PP1-binding motif in the Cdc25 N-terminus. We have also found that 14-3-3 binding to phospho-Ser287 protects Cdc25 from premature dephosphorylation. Upon entry into M phase, 14-3-3 removal from Cdc25 precedes Ser287 dephosphorylation, suggesting the existence of a phosphatase- independent pathway for 14-3-3 removal from Cdc25. We show here that this dissociation of 14-3-3 from Cdc25 requires the activity of the cyclin-dependent kinase Cdk2, providing a molecular explanation for the previously reported requirement for Cdk2 in promoting mitotic entry. Collectively, our data clarify several steps important for Cdc25 activation and provide new insight into the role of PP1 in Cdc2 activation and mitotic entry.
Figures




References
-
- Bulavin D.V. et al. (2003) Dual phosphorylation controls Cdc25 phosphatases and mitotic entry. Nat. Cell Biol., 5, 545–551. - PubMed
-
- Cohen P.T. (2002) Protein phosphatase 1-targeted in many directions. J. Cell Sci., 115, 241–256. - PubMed
-
- Coleman T.R. and Dunphy,W.G. (1994) Cdc2 regulatory factors. Curr. Opin. Cell Biol., 6, 877–882. - PubMed
-
- Connor J.H., Quan,H., Oliver,C. and Shenolikar,S. (1998) Inhibitor-1, a regulator of protein phosphatase 1 function. Methods Mol. Biol., 93, 41–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

