scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila
- PMID: 14592975
- PMCID: PMC275405
- DOI: 10.1093/emboj/cdg548
scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila
Abstract
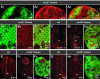
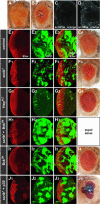
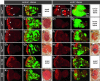
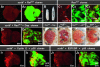
Cancer is a multistep process involving cooperation between oncogenic or tumor suppressor mutations and interactions between the tumor and surrounding normal tissue. Here we present the first description of cooperative tumorigenesis in Drosophila, by using a system that mimics the development of tumors in mammals. We have used the MARCM system to generate mutant clones of the apical-basal cell polarity tumor suppressor gene, scribble, in the context of normal tissue. We show that scribble mutant clones in the eye disc exhibit ectopic expression of cyclin E and ectopic cell cycles, but do not overgrow due to increased cell death mediated by the JNK pathway and the surrounding wild-type tissue. In contrast, when oncogenic Ras or Notch is expressed within the scribble mutant clones, cell death is prevented and neoplastic tumors develop. This demonstrates, for the first time in Drosophila, that activated alleles of Ras and Notch can act as cooperating oncogenes in the development of epithelial tumors, and highlights the importance of epithelial polarity regulators in restraining oncogenes and preventing tumor formation.
Figures

References
-
- Adachi-Yamada T. and O’Connor,M.B. (2002) Morphogenetic apoptosis: a mechanism for correcting discontinuities in morphogen gradients. Dev. Biol., 251, 74–90. - PubMed
-
- Adachi-Yamada T., Fujimura-Kamada,K., Nishida,Y. and Matsumoto,K. (1999) Distortion of proximodistal information causes JNK-dependent apoptosis in Drosophila wing. Nature, 400, 166–169. - PubMed
-
- Agrawal N., Kango,M., Mishra,A. and Sinha,P. (1995) Neoplastic transformation and aberrant cell–cell interactions in genetic mosaics of lethal(2)giant larvae (lgl), a tumor suppressor gene of Drosophila. Dev. Biol., 172, 218–229. - PubMed
-
- Bergmann A., Agapite,J., McCall,K.A. and Steller,H. (1998) The Drosophila gene hid is a direct molecular target of Ras-dependent survival signaling. Cell, 95, 331–341. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

