Feedback control of the protein kinase TAK1 by SAPK2a/p38alpha
- PMID: 14592977
- PMCID: PMC275409
- DOI: 10.1093/emboj/cdg552
Feedback control of the protein kinase TAK1 by SAPK2a/p38alpha
Abstract
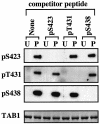
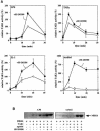
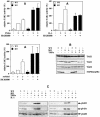
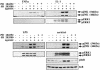
TAB1, a subunit of the kinase TAK1, was phosphorylated by SAPK2a/p38alpha at Ser423, Thr431 and Ser438 in vitro. TAB1 became phosphorylated at all three sites when cells were exposed to cellular stresses, or stimulated with tumour necrosis factor-alpha (TNF-alpha), interleukin-1 (IL-1) or lipopolysaccharide (LPS). The phosphorylation of Ser423 and Thr431 was prevented if cells were pre-incubated with SB 203580, while the phosphorylation of Ser438 was partially inhibited by PD 184352. Ser423 is the first residue phosphorylated by SAPK2a/p38alpha that is not followed by proline. The activation of TAK1 was enhanced by SB 203580 in LPS-stimulated macrophages, and by proinflammatory cytokines or osmotic shock in epithelial KB cells or embryonic fibroblasts. The activation of TAK1 by TNF-alpha, IL-1 or osmotic shock was also enhanced in embryonic fibroblasts from SAPK2a/p38alpha-deficient mice, while incubation of these cells with SB 203580 had no effect. Our results suggest that TAB1 participates in a SAPK2a/p38alpha-mediated feedback control of TAK1, which not only limits the activation of SAPK2a/p38alpha but synchronizes its activity with other signalling pathways that lie downstream of TAK1 (JNK and IKK).
Figures







References
-
- Adams R.H. et al. (2000) Essential role of p38α MAP kinase in placental but not embryonic cardiovascular development. Mol. Cell, 6, 109–116. - PubMed
-
- Alessi D.R., Smythe,C. and Keyse,S.M. (1993) The human CL100 gene encodes a Tyr/Thr-protein phosphatase which potently and specifically inactivates MAP kinase and suppresses its activation by oncogenic ras in Xenopus oocyte extracts. Oncogene, 8, 2015–2020, - PubMed
-
- Alessi D.R., Cohen,P., Ashworth,A., Cowley,S., Leevers,S.J. and Marshall,C.J. (1995) Assay and expression of mitogen-activated protein kinase, MAP kinase kinase and Raf. Methods Enzymol., 255, 279–290. - PubMed
-
- Alonso G., Ambrosino,C., Jones,M. and Nebreda,A.R. (2000) Differential activation of p38 mitogen-activated protein kinase isoforms depending on signal strength. J. Biol. Chem., 275, 40641–40648. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

