C/EBPbeta: a major PML-RARA-responsive gene in retinoic acid-induced differentiation of APL cells
- PMID: 14592978
- PMCID: PMC275413
- DOI: 10.1093/emboj/cdg556
C/EBPbeta: a major PML-RARA-responsive gene in retinoic acid-induced differentiation of APL cells
Abstract
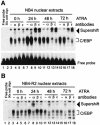
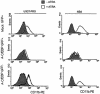
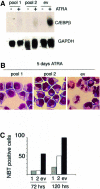
In acute promyelocytic leukemia (APL), the translocation t(15;17) induces a block at the promyelocytic stage of differentiation in an all-trans-retinoic acid (ATRA)-responsive manner. Here we report that upon treatment with ATRA, t(15;17) cells (NB4) reveal a very rapid increase in protein level and binding activity of C/EBPbeta, a C/EBP family member, which was not observed in an ATRA-resistant NB4 cell line. We further provide evidence that ATRA mediates a direct increase of C/EBPbeta, only in PML-RARA (promyelocytic leukemia-retinoic acid receptor alpha)-expressing cells. In addition, transactivation experiments indicate that the PML-RARA fusion protein, but not PML-RARA mutants defective in transactivation, strongly transactivates the C/EBPbeta promoter. These results suggest that PML-RARA mediates ATRA-induced C/EBPbeta expression. Finally, we demonstrate the importance of C/EBPbeta in granulocytic differentiation. We show that not only does C/EBPbeta induce granulocytic differentiation of non-APL myeloid cell lines independent of addition of ATRA or other cytokines, but also that C/EBPbeta induction is required during ATRA-induced differentiation of APL cells. Taken together, C/EBPbeta is an ATRA-dependent PML-RARA target gene involved in ATRA-induced differentiation of APL cells.
Figures
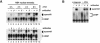




References
-
- Borrow J., Goddard,A.D., Sheer,D. and Solomon,E. (1990) Molecular analysis of acute promyelocytic leukemia breakpoint cluster region on chromosome 17. Science, 249, 1577–1580. - PubMed
-
- Castaigne S., Chomienne,C., Daniel,M.T., Berger,R., Miclea,J.M., Ballerini,P. and Degos,L. (1990) Retinoic acids in treatment of acute promyelocytic leukemia. Nouv. Rev. Fr. Hematol., 32, 36–38. - PubMed
-
- Chih D.Y., Chumakov,A.M., Park,D.J., Silla,A.G. and Koeffler,H.P. (1997) Modulation of mRNA expression of a novel human myeloid-selective CCAAT/enhancer binding protein gene (C/EBPε). Blood, 90, 2987–2994. - PubMed
-
- de The H., Chomienne,C., Lanotte,M., Degos,L. and Dejean,A. (1990) The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor α gene to a novel transcribed locus. Nature, 347, 558–561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

