Descriptive survey of non-commercial randomised controlled trials in the United Kingdom, 1980-2002
- PMID: 14593034
- PMCID: PMC261654
- DOI: 10.1136/bmj.327.7422.1017
Descriptive survey of non-commercial randomised controlled trials in the United Kingdom, 1980-2002
Abstract
Objectives: To describe the characteristics of randomised controlled trials supported by the main non-commercial sources of funding in the United Kingdom between 1980 and 2002.
Design: Descriptive survey.
Setting: Randomised controlled trials funded by the Medical Research Council, NHS research and development programme, Department of Health, Chief Scientist Office in Scotland, and medical research charities.
Participants: 1464 randomised controlled trials supported by the main non-commercial sources of funding.
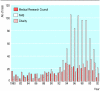
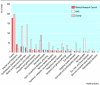
Results: Support for randomised controlled trials by the main sources of non-commercial funding in the United Kingdom has fallen in recent years, without any concomitant increase in the sample sizes of these studies. Drug trials in a limited range of health problems have dominated among the studies supported by the Medical Research Council and medical research charities. Until recently, the NHS research and development programme supported randomised controlled trials of various healthcare interventions, in a wide range of health problems, but between 1999 and 2002 many of the subprogrammes that had commissioned trials were discontinued.
Conclusions: The future of non-commercial randomised controlled trials in the United Kingdom has been threatened by the discontinuation or demise of national and regional NHS research and development programmes. Support also seems to be declining from the Medical Research Council and the medical research charities. It is unclear what the future holds for randomised controlled trials that address issues of no interest to industry but are of great importance to patients and practitioners.
Figures

Comment in
-
Non-commercial randomised clinical trials need money for meetings and travel expenses.BMJ. 2003 Dec 6;327(7427):1349. doi: 10.1136/bmj.327.7427.1349. BMJ. 2003. PMID: 14656866 Free PMC article. No abstract available.
-
Academic medicine: time for reinvention: MRC aims to give NHS evidence from randomised clinical trials.BMJ. 2004 Jan 3;328(7430):49; discussion 49. doi: 10.1136/bmj.328.7430.49. BMJ. 2004. PMID: 14703562 Free PMC article. No abstract available.
References
-
- Medical Research Council. Clinical trials for tomorrow. London: MRC, 2002: 4. (Consultation document.)
-
- Medical Research Council. Report on the clinical trials for tomorrow review. London: MRC, 2003.
-
- Chalmers I, Rounding C, Lock K. UK RCT Registration Project: final report. Oxford: UK Cochrane Centre, 2000:appendices 3.a.i, 3.b.1, 3.c.i.
-
- Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized trial. Lancet 2002;360: 1267-74. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
