Dictyostelium stress-activated protein kinase alpha, a novel stress-activated mitogen-activated protein kinase kinase kinase-like kinase, is important for the proper regulation of the cytoskeleton
- PMID: 14593072
- PMCID: PMC266770
- DOI: 10.1091/mbc.e03-01-0039
Dictyostelium stress-activated protein kinase alpha, a novel stress-activated mitogen-activated protein kinase kinase kinase-like kinase, is important for the proper regulation of the cytoskeleton
Abstract
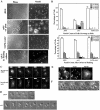
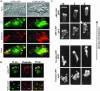
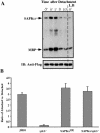
Mitogen-activated protein kinase cascades regulate various cellular functions, including growth, cell differentiation, development, and stress responses. We have identified a new Dictyostelium kinase (stress-activated protein kinase [SAPK]alpha), which is related to members of the mixed lineage kinase class of mitogen-activated protein kinase kinases. SAPKalpha is activated by osmotic stress, heat shock, and detachment from the substratum and by a membrane-permeable cGMP analog, a known regulator of stress responses in Dictyostelium. SAPKalpha is important for cellular resistance to stresses, because SAPKalpha null cells exhibit reduced viability in response to osmotic stress. We found that SAPKalpha mutants affect cellular processes requiring proper regulation of the actin cytoskeleton, including cell motility, morphogenesis, cytokinesis, and cell adhesion. Overexpression of SAPKalpha results in highly elevated basal and chemoattractant-stimulated F-actin levels and strong aggregation and developmental defects, including a failure to polarize and chemotax, and abnormal morphogenesis. These phenotypes require a kinase-active SAPKalpha. SAPKalpha null cells exhibit reduced chemoattractant-stimulated F-actin levels, cytokinesis, developmental and adhesion defects, and a motility defect that is less severe than that exhibited by SAPKalpha-overexpressing cells. SAPKalpha colocalizes with F-actin in F-actin-enriched structures, including membrane ruffles and pseudopodia during chemotaxis. Although SAPKalpha is required for these F-actin-mediated processes, it is not detectably activated in response to chemoattractant stimulation.
Figures






Similar articles
-
PAKa, a putative PAK family member, is required for cytokinesis and the regulation of the cytoskeleton in Dictyostelium discoideum cells during chemotaxis.J Cell Biol. 1999 Nov 1;147(3):559-76. doi: 10.1083/jcb.147.3.559. J Cell Biol. 1999. PMID: 10545500 Free PMC article.
-
The Dictyostelium LIM domain-containing protein LIM2 is essential for proper chemotaxis and morphogenesis.Mol Biol Cell. 2000 Apr;11(4):1275-91. doi: 10.1091/mbc.11.4.1275. Mol Biol Cell. 2000. PMID: 10749929 Free PMC article.
-
The Dictyostelium MAP kinase kinase DdMEK1 regulates chemotaxis and is essential for chemoattractant-mediated activation of guanylyl cyclase.EMBO J. 1997 Jul 16;16(14):4317-32. doi: 10.1093/emboj/16.14.4317. EMBO J. 1997. PMID: 9250676 Free PMC article.
-
The regulation of myosin II in Dictyostelium.Eur J Cell Biol. 2006 Sep;85(9-10):969-79. doi: 10.1016/j.ejcb.2006.04.004. Epub 2006 Jun 30. Eur J Cell Biol. 2006. PMID: 16814425 Review.
-
The actin cytoskeleton of Dictyostelium: a story told by mutants.J Cell Sci. 2000 Mar;113 ( Pt 5):759-66. doi: 10.1242/jcs.113.5.759. J Cell Sci. 2000. PMID: 10671366 Review.
Cited by
-
Rap1 controls cell adhesion and cell motility through the regulation of myosin II.J Cell Biol. 2007 Mar 26;176(7):1021-33. doi: 10.1083/jcb.200607072. Epub 2007 Mar 19. J Cell Biol. 2007. PMID: 17371831 Free PMC article.
-
Regulation of Rap1 activity by RapGAP1 controls cell adhesion at the front of chemotaxing cells.J Cell Biol. 2007 Dec 3;179(5):833-43. doi: 10.1083/jcb.200705068. Epub 2007 Nov 26. J Cell Biol. 2007. PMID: 18039932 Free PMC article.
-
The N-terminus of Dictyostelium Scar interacts with Abi and HSPC300 and is essential for proper regulation and function.Mol Biol Cell. 2007 May;18(5):1609-20. doi: 10.1091/mbc.e06-06-0518. Epub 2007 Feb 21. Mol Biol Cell. 2007. PMID: 17314411 Free PMC article.
-
Intramolecular activation mechanism of the Dictyostelium LRRK2 homolog Roco protein GbpC.J Biol Chem. 2008 Oct 31;283(44):30412-20. doi: 10.1074/jbc.M804265200. Epub 2008 Aug 14. J Biol Chem. 2008. PMID: 18703517 Free PMC article.
-
Evolutionary origin of cAMP-based chemoattraction in the social amoebae.Proc Natl Acad Sci U S A. 2005 May 3;102(18):6385-90. doi: 10.1073/pnas.0502238102. Epub 2005 Apr 25. Proc Natl Acad Sci U S A. 2005. PMID: 15851658 Free PMC article.
References
-
- Aizawa, H., Katadae, M., Maruya, M., Sameshima, M., Murakami-Murofushi, K., and Yahara, I. (1999). Hyperosmotic stress-induced reorganization of actin bundles in Dictyostelium cells over-expressing cofilin. Genes Cells 4, 311-324. - PubMed
-
- Aubry, L., and Firtel, R. (1999). Integration of signaling networks that regulate Dictyostelium differentiation. Annu. Rev. Cell Dev. Biol. 15, 469-517. - PubMed
-
- Canman, C.E., and Kastan, M.B. (1996). Signal transduction. Three paths to stress relief. Nature 384, 213-214. - PubMed
-
- Chang, L., and Karin, M. (2001). Mammalian MAP kinase signalling cascades. Nature 410, 37-40. - PubMed
-
- Chaudoir, B.M., Kowalczyk, P.A., and Chisholm, R.L. (1999). Regulatory light chain mutations affect myosin motor function and kinetics. J. Cell Sci. 112, 1611-1620. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

