Posttranscriptional control of the competence factor betaFTZ-F1 by juvenile hormone in the mosquito Aedes aegypti
- PMID: 14593204
- PMCID: PMC263810
- DOI: 10.1073/pnas.2234416100
Posttranscriptional control of the competence factor betaFTZ-F1 by juvenile hormone in the mosquito Aedes aegypti
Abstract
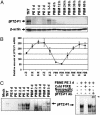
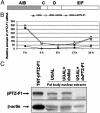
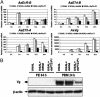
In anautogenous mosquitoes, vitellogenesis, which includes production of yolk protein precursors, requires blood feeding. Consequently, mosquitoes transmit many diseases. Understanding the molecular mechanisms of vitellogenesis regulation will contribute significantly to vector control strategies. Newly emerged Aedes aegypti females require 3 days before becoming competent to activate vitellogenesis in response to a blood-meal-initiated, elevated titer of 20-hydroxyecdysone (20E). An orphan nuclear receptor gene betaFTZ-F1 is transcribed in the fat body of newly emerged mosquito females; however, the betaFTZ-F1 protein is only found 3 days later. Dramatically increased titer of the juvenile hormone III (JH III) is essential for the acquisition of 20E competence. In vitro fat body culture experiments have shown that betaFTZ-F1 protein appears after exposure to JH III. Injection of double-stranded RNA complementary to betaFTZ-F1 into newly emerged females attenuated expression of the early genes EcR-B, E74B, and E75A and the target YPP gene Vg, in response to a blood meal. Thus, betaFTZ-F1 is indeed the factor defining the acquisition of competence to 20E in the mosquito fat body. Moreover, this is achieved through JH III-mediated posttranscriptional control of betaFTZ-F1.
Figures



Similar articles
-
Conserved molecular mechanism for the stage specificity of the mosquito vitellogenic response to ecdysone.Dev Biol. 2000 Aug 1;224(1):96-110. doi: 10.1006/dbio.2000.9792. Dev Biol. 2000. PMID: 10898964
-
The early gene Broad is involved in the ecdysteroid hierarchy governing vitellogenesis of the mosquito Aedes aegypti.J Mol Endocrinol. 2004 Dec;33(3):743-61. doi: 10.1677/jme.1.01531. J Mol Endocrinol. 2004. PMID: 15591032
-
Juvenile hormone-regulated alternative splicing of the taiman gene primes the ecdysteroid response in adult mosquitoes.Proc Natl Acad Sci U S A. 2018 Aug 14;115(33):E7738-E7747. doi: 10.1073/pnas.1808146115. Epub 2018 Jul 30. Proc Natl Acad Sci U S A. 2018. PMID: 30061397 Free PMC article.
-
Regulatory Mechanisms of Vitellogenesis in Insects.Front Cell Dev Biol. 2021 Jan 28;8:593613. doi: 10.3389/fcell.2020.593613. eCollection 2020. Front Cell Dev Biol. 2021. PMID: 33634094 Free PMC article. Review.
-
Molecular biology of mosquito vitellogenesis: from basic studies to genetic engineering of antipathogen immunity.Insect Biochem Mol Biol. 2002 Oct;32(10):1275-86. doi: 10.1016/s0965-1748(02)00090-5. Insect Biochem Mol Biol. 2002. PMID: 12225918 Review.
Cited by
-
Juvenile hormone-activated phospholipase C pathway enhances transcriptional activation by the methoprene-tolerant protein.Proc Natl Acad Sci U S A. 2015 Apr 14;112(15):E1871-9. doi: 10.1073/pnas.1423204112. Epub 2015 Mar 30. Proc Natl Acad Sci U S A. 2015. PMID: 25825754 Free PMC article.
-
Role of Groucho and Groucho1-like in Regulating Metamorphosis and Ovary Development in Nilaparvata lugens (Stål).Int J Mol Sci. 2022 Jan 21;23(3):1197. doi: 10.3390/ijms23031197. Int J Mol Sci. 2022. PMID: 35163119 Free PMC article.
-
Endocrine Regulation of Lifespan in Insect Diapause.Front Physiol. 2022 Feb 15;13:825057. doi: 10.3389/fphys.2022.825057. eCollection 2022. Front Physiol. 2022. PMID: 35242054 Free PMC article. Review.
-
Juvenile hormone and its receptor methoprene-tolerant promote ribosomal biogenesis and vitellogenesis in the Aedes aegypti mosquito.J Biol Chem. 2017 Jun 16;292(24):10306-10315. doi: 10.1074/jbc.M116.761387. Epub 2017 Apr 26. J Biol Chem. 2017. PMID: 28446607 Free PMC article.
-
Nuclear Receptor FTZ-F1 Controls Locust Molt by Regulating the Molting Process of Locusta migratoria.Insects. 2024 Mar 29;15(4):237. doi: 10.3390/insects15040237. Insects. 2024. PMID: 38667367 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

