Generation of hair cells by stepwise differentiation of embryonic stem cells
- PMID: 14593207
- PMCID: PMC263842
- DOI: 10.1073/pnas.2334503100
Generation of hair cells by stepwise differentiation of embryonic stem cells
Abstract
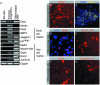
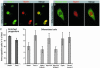
The increase in life expectancy is accompanied by the growing burden of chronic diseases. Hearing loss is perhaps the most prevalent of all chronic diseases. In addition to age-related hearing loss, a substantial number of cases of audiological impairment are either congenital in nature or acquired during childhood. The permanence of hearing loss is mainly due to the inability of the cochlear sensory epithelium to replace lost mechanoreceptor cells, or hair cells. Generation of hair cells from a renewable source of progenitors that can be transplanted into damaged inner ears is a principal requirement for potential cell replacement therapy in this organ. Here, we present an experimental protocol that enables us to routinely create inner ear progenitors from murine embryonic stem cells in vitro. These progenitors express a comprehensive set of marker genes that define the developing inner ear, in particular the organ's developing sensory patches. We further demonstrate that cells that express markers characteristic of hair cells differentiate from embryonic stem cell-derived progenitors. Finally, we show that these progenitors integrate into the developing inner ear at sites of epithelial injury and that integrated cells start expressing hair cell markers and display hair bundles when situated in cochlear or vestibular sensory epithelia in vivo.
Figures

References
-
- Lee, S. H., Lumelsky, N., Studer, L., Auerbach, J. M. & McKay, R. D. (2000) Nat. Biotechnol. 18, 675-679. - PubMed
-
- Lumelsky, N., Blondel, O., Laeng, P., Velasco, I., Ravin, R. & McKay, R. (2001) Science 292, 1389-1394. - PubMed
-
- Doetschman, T. C., Eistetter, H., Katz, M., Schmidt, W. & Kemler, R. (1985) J. Embryol. Exp. Morphol. 87, 27-45. - PubMed
-
- Wichterle, H., Lieberam, I., Porter, J. A. & Jessell, T. M. (2002) Cell 110, 385-397. - PubMed
-
- Pickles, J. O. & van Heumen, W. R. (1997) Dev. Neurosci. 19, 476-487. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

