Src kinase activation by direct interaction with the integrin beta cytoplasmic domain
- PMID: 14593208
- PMCID: PMC263791
- DOI: 10.1073/pnas.2336149100
Src kinase activation by direct interaction with the integrin beta cytoplasmic domain
Abstract
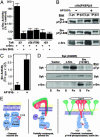
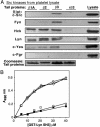
Src tyrosine kinases transmit integrin-dependent signals pivotal for cell movement and proliferation. Here, we establish a mechanism for Src activation by integrins. c-Src is shown to bind constitutively and selectively to beta3 integrins through an interaction involving the c-Src SH3 domain and the carboxyl-terminal region of the beta3 cytoplasmic tail. Clustering of beta3 integrins in vivo activates c-Src and induces phosphorylation of Tyr-418 in the c-Src activation loop, a reaction essential for adhesion-dependent phosphorylation of Syk, a c-Src substrate. Unlike c-Src, Hck, Lyn, and c-Yes bind more generally to beta1A, beta2, and beta3 cytoplasmic tails. These results invoke a model whereby Src is primed for activation by direct interaction with an integrin beta tail, and integrin clustering stabilizes activated Src by inducing intermolecular autophosphorylation. The data provide a paradigm for integrin regulation of Src and a molecular basis for the similar functional defects of osteoclasts or platelets from mice lacking beta3 integrins or c-Src.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

