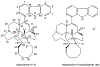
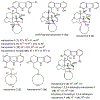
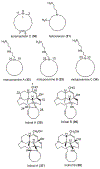
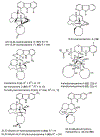
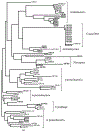
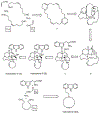
The manzamine alkaloids
- PMID: 14593858
- PMCID: PMC10228634
- DOI: 10.1016/s0099-9598(03)60004-0
The manzamine alkaloids
Figures




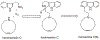
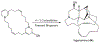
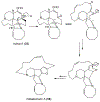
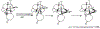
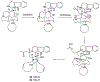
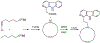
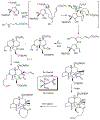
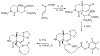
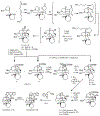
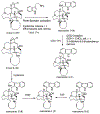
References
-
- Sakai R, Higa T, Jefford CW, and Bernardinelli G, J. Am. Chem. Soc 108, 6404–6405 (1986).
-
- Tsuda M and Kobayashi J, Heterocycles 46, 765–794 (1997).
- Baker BJ, Beta-carboline and isoquinoline alkaloids from marine organisms, “Alkaloids: Chemical and Biological Perspectives” (Pelletier W, ed.), vol. 10, pp. 357–407. Pergamon, Oxford, 1996.
- Ihara M and Fukumoto K, Nat. Prod. Rep 12, 277–301 (1997).
-
- Whitehead R, Annu. Rep. Prog. Chem. Sect. B 95, 183–205 (1999).
-
- Urban S, Hickford SJH, Blunt JW, Munro MHG, Curr. Org. Chem 4, 765–807 (2000).
- Higa T, Ohtani II, and Tanaka J, ACS Symposium Series, Vol. 745 (Natural and Selected Synthetic Toxins; ), 12–21 (2000).
- Rodríguez J, Studies in Natural Products Chemistry, Vol. 24 [Bioactive Natural Products (Part E)], 573–681 (2000).
- Higa T, Tanaka J, Ohtani II, Musman M, Roy MC, and Kuroda I, Pure Appl. Chem 73, 589–593 (2000).
- Higa T, Tanaka J, and Tan LT, Cytotoxic Macrocycles from Marine Sponges, New Trends Nat. Prod. Chem (Int. Symp. Nat. Prod. Chem., 6th, Meeting Data 1996) (Atta-ur-Rahman and Choudhary MI, eds.), pp. 109–120. Harwood, Amsterdam, 1998. CA129: 185141.
- Barriault L, and Paquette LA, Chemtracts 12, 76–281 (1999). CA131: 73818.
-
- Baldwin JE and Whitehead RC, Tetrahedron Lett. 33, 2059–2062 (1992).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
