Ovine feto-placental metabolism
- PMID: 14594988
- PMCID: PMC1664765
- DOI: 10.1113/jphysiol.2003.054577
Ovine feto-placental metabolism
Abstract
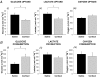
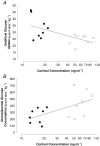
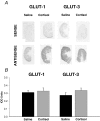
Fetal growth depends on the transplacental nutrient supply, which, in turn, is determined partially by the consumption and production of nutrients by the uteroplacental tissues. In fetal sheep, the rates of growth and umbilical glucose uptake decline coincidently towards term in parallel with the normal prepartum rise in plasma cortisol. While cortisol is known to reduce growth in fetal sheep, its effects on the uteroplacental handling and delivery of nutrients remain unknown. Hence, this study, quantified the rates of umbilical uptake and uteroplacental consumption of nutrients in preterm fetuses infused with cortisol for 5 days to mimic the prepartum cortisol surge. Umbilical uptakes of glucose and lactate, but not oxygen, were significantly lower in cortisol- than saline-infused fetuses, irrespective of whether values were expressed as absolute or weight-specific rates. The rate of uteroplacental consumption of glucose, but not oxygen, was significantly higher in cortisol- than saline-infused animals. Absolute rates of uteroplacental lactate production were lower in cortisol-infused animals. When all data were combined, fetal plasma cortisol levels were positively correlated to uteroplacental glucose consumption and inversely related to umbilical glucose uptake. Cortisol treatment had no apparent effect on placental mRNA expression for the glucose transporters, GLUT-1 and GLUT-3. The results demonstrate that cortisol is physiological regulator of uteroplacental metabolism and nutrient delivery to the sheep fetus. These observations have important implications for fetal growth both in late gestation and during adverse intrauterine conditions, which raise fetal cortisol levels earlier in gestation.
Figures
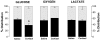
References
-
- Aldoretta PW, Hay WW. Effect of glucose supply on ovine uteroplacental glucose metabolism. Am J Physiol. 1999;277(4):R947–R958. - PubMed
-
- Barbera A, Wilkening RB, Teng C, Battaglia FC, Meschia G. Metabolic alterations in the fetal heaptic and umbilical circulations during glucocorticoid-induced parturition in sheep. Ped Res. 1997;41:242–248. - PubMed
-
- Battaglia FC. An Introduction to Fetal Physiology. Orlando: Academic Press; 1986.
-
- Bell AW, Ehrhardt RA. Placental Regulation of Nutrient Partitioning During Pregnancy. In: Bray GA, Hansel W, Ryan DH, editors. Nutrition & Reproduction. Baton Rouge: Louisiana. State University Press; 1998. pp. 229–254.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

