RNA interference-mediated knockdown of a GATA factor reveals a link to anautogeny in the mosquito Aedes aegypti
- PMID: 14595016
- PMCID: PMC263821
- DOI: 10.1073/pnas.2235649100
RNA interference-mediated knockdown of a GATA factor reveals a link to anautogeny in the mosquito Aedes aegypti
Abstract
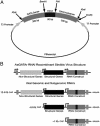
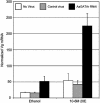
Blood feeding tightly regulates the reproductive cycle in anautogenous mosquitoes. Vitellogenesis (the synthesis of yolk protein precursors) is a key event in the mosquito reproductive cycle and is activated in response to a blood meal. Before blood feeding, Aedes aegypti is in a state of reproductive arrest during which the yolk protein precursor genes (YPPs) are repressed. The regulatory region of the major YPP gene vitellogenin (Vg) has multiple GATA-binding sites required for the high expression level of this gene. However, a GATA factor (AaGATAr) likely acts as a repressor, preventing activation of this gene before a blood meal. Here we report in vivo data confirming the role of AaGATAr as a repressor of the Vg gene at the state of previtellogenic arrest. Using an RNA interference (RNAi)-mediated technique in conjunction with the Sindbis viral expression system, we show that knockdown of the AaGATAr gene results in an increased basal level of expression of the Vg gene and an elevated response to the steroid hormone 20-hydroxyecdysone in mosquitoes in a state of arrest. These experiments have revealed a component in the molecular mechanism by which anautogeny is maintained in A. aegypti.
Figures



References
-
- Eberle, M. W. & Reisen, W. K. (1986) J. Am. Mosq. Control Assoc. 2, 38-43. - PubMed
-
- Brust, R. A. (1991) J. Med. Entomol. 28, 847-853. - PubMed
-
- Reisen, W. K., Milby, M. M. & Bock, M. E. (1986) Mosq. News 44, 385-395.
-
- Su, T. & Mulla, M. S. (1997) J. Med. Entomol. 34, 68-73. - PubMed
-
- Klowden, M. J. & Chambers, G. M. (1992) J. Med. Entomol. 29, 467-471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

