Pyrin binds the PSTPIP1/CD2BP1 protein, defining familial Mediterranean fever and PAPA syndrome as disorders in the same pathway
- PMID: 14595024
- PMCID: PMC263843
- DOI: 10.1073/pnas.2135380100
Pyrin binds the PSTPIP1/CD2BP1 protein, defining familial Mediterranean fever and PAPA syndrome as disorders in the same pathway
Abstract
Pyrin, the familial Mediterranean fever protein, is found in association with the cytoskeleton in myeloid/monocytic cells and modulates IL-1beta processing, NF-kappaB activation, and apoptosis. These effects are mediated in part through cognate interactions with the adaptor protein ASC, which shares an N-terminal motif with pyrin. We sought additional upstream regulators of inflammation by using pyrin as the bait in yeast two-hybrid assays. We now show that proline serine threonine phosphatase-interacting protein [PSTPIP1, or CD2-binding protein 1 (CD2BP1)], a tyrosine-phosphorylated protein involved in cytoskeletal organization, also interacts with pyrin. Recently, PSTPIP1/CD2BP1 mutations were shown to cause the syndrome of pyogenic arthritis, pyoderma gangrenosum, and acne (PAPA), a dominantly inherited autoinflammatory disorder mediated predominantly by granulocytes. Endogenous PSTPIP1/CD2BP1 and pyrin are coexpressed in monocytes and granulocytes and can be coimmunoprecipitated from THP-1 cells. The B box segment of pyrin was necessary and the B box/coiled-coil segment sufficient for this interaction, whereas the SH3 and coiled-coil domains of PSTPIP1/CD2BP1 were both necessary, but neither was sufficient, for pyrin binding. The Y344F PSTPIP1/CD2BP1 mutation, which blocks tyrosine phosphorylation, was associated with a marked reduction in pyrin binding in pervanadate-treated cells. PAPA-associated A230T and E250Q PSTPIP1/CD2BP1 mutations markedly increased pyrin binding as assayed by immunoprecipitation and, relative to WT, these mutants were hyperphosphorylated when coexpressed with c-Abl kinase. Consistent with the hypothesis that these mutations exert a dominant-negative effect on the previously reported activity of pyrin, we found increased IL-1beta production by peripheral blood leukocytes from a clinically active PAPA patient with the A230T PSTPIP1/CD2BP1 mutation and in cell lines transfected with both PAPA-associated mutants.
Figures
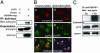




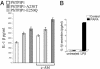
References
-
- Galon, J., Aksentijevich, I., McDermott, M. F., O'Shea, J. J. & Kastner, D. L. (2000) Curr. Opin. Immunol. 12, 479-486. - PubMed
-
- Kastner, D. L. (2003) in Rheumatology, eds. Hochberg, M. C., Silman, A. J., Smolen J. S., Weinblatt, M. E. & Weisman, M. H. (Mosby, Edinburgh), 3rd Ed., pp. 1717-1734.
-
- International FMF Consortium (1997) Cell 90, 797-807. - PubMed
-
- French FMF Consortium (1997) Nat. Genet. 17, 25-31. - PubMed
-
- Centola, M., Wood, G., Frucht, D. M., Galon, J., Aringer, M., Farrell, C., Kingma, D. W., Horwitz, M. E., Mansfield, E., Holland, S. M., et al. (2000) Blood 95, 3223-3231. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

