N-desmethylclozapine, an allosteric agonist at muscarinic 1 receptor, potentiates N-methyl-D-aspartate receptor activity
- PMID: 14595031
- PMCID: PMC263872
- DOI: 10.1073/pnas.1835612100
N-desmethylclozapine, an allosteric agonist at muscarinic 1 receptor, potentiates N-methyl-D-aspartate receptor activity
Abstract
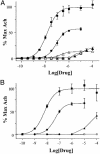
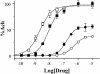
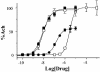
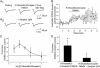
The molecular and neuronal substrates conferring on clozapine its unique and superior efficacy in the treatment of schizophrenia remain elusive. The interaction of clozapine with many G protein-coupled receptors is well documented but less is known about its biologically active metabolite, N-desmethylclozapine. Recent clinical and preclinical evidences of the antipsychotic activity of the muscarinic agonist xanomeline prompted us to investigate the effects of N-desmethylclozapine on cloned human M1-M5 muscarinic receptors. N-desmethylclozapine preferentially bound to M1 muscarinic receptors with an IC50 of 55 nM and was a more potent partial agonist (EC50, 115 nM and 50% of acetylcholine response) at this receptor than clozapine. Furthermore, pharmacological and site-directed mutagenesis studies suggested that N-desmethylclozapine preferentially activated M1 receptors by interacting with a site that does not fully overlap with the acetylcholine orthosteric site. As hypofunction of N-methyl-d-aspartate (NMDA) receptor-driven neuronal ensembles has been implicated in psychotic disorders, the neuronal activity of N-desmethylclozapine was electrophysiologically investigated in hippocampal rat brain slices. N-desmethylclozapine was shown to dose-dependently potentiate NMDA receptor currents in CA1 pyramidal cells by 53% at 100 nM, an effect largely mediated by activation of muscarinic receptors. Altogether, our observations provide direct evidence that the brain penetrant metabolite N-desmethylclozapine is a potent, allosteric agonist at human M1 receptors and is able to potentiate hippocampal NMDA receptor currents through M1 receptor activation. These observations raise the possibility that N-desmethylclozapine contributes to clozapine's clinical activity in schizophrenics through modulation of both muscarinic and glutamatergic neurotransmission.
Figures
References
-
- Kane, J, Honigfeld, G., Singer, J. & Meltzer, H. (1988) Arch. Gen. Psychiatry 45, 789-796. - PubMed
-
- Volavka, J., Czobor, P., Lindemayer, J.-P., Citrome, L., McEvoy, J. P., Cooper, T. B., Chakos, M. & Lieberman, J. A. (2002) Am. J. Psychiatry 159, 255-262. - PubMed
-
- Meltzer, H. Y. & McGurk, S. R. (1999) Schizophrenia Bull. 25, 233-255. - PubMed
-
- Meltzer, H. Y. & Okayli, G. (1995) Am. J. Psychiatry 152, 183-190. - PubMed
-
- Lindenmayer, J.-P., Iskander, A., Park, M., Apergi, F.-S., Czobor, P., Smith, R. & Allen, D. (1998) J. Clin. Psychiatry 59, 521-527. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

