Epithelial cell polarity alters Rho-GTPase responses to Pseudomonas aeruginosa
- PMID: 14595106
- PMCID: PMC329196
- DOI: 10.1091/mbc.e03-08-0559
Epithelial cell polarity alters Rho-GTPase responses to Pseudomonas aeruginosa
Abstract
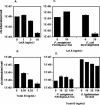
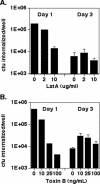
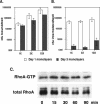
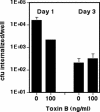
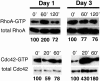
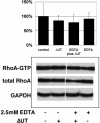
Pseudomonas aeruginosa is an opportunistic human pathogen that preferentially infects damaged epithelial tissues. Previous studies have failed to distinguish whether the increased susceptibility of injured epithelium results from the loss of cell polarity or increased access to the basolateral surface. We have used confluent monolayers of Madin-Darby canine kidney (MDCK) cells cultured on porous filter supports for 1-3 d as a model system to investigate whether the differentiation state of a polarized model epithelium affected the response of epithelial cells to this pathogen. Confluent incompletely polarized MDCK cell monolayers (day 1) efficiently internalized apically applied P. aeruginosa via a pathway that required actin polymerization and activation of Rho-family GTPases and was accompanied by an increase in the amount of activated RhoA. In contrast, P. aeruginosa entry into highly polarized MDCK monolayers (day 3) was 10- to 100-fold less efficient and was insensitive to inhibitors of actin polymerization or of Rho-family GTPase activation. There was no activation of RhoA; instead, Cdc42-GTP levels increased significantly. Basolateral infection of highly polarized MDCK monolayers was less efficient and insensitive to Clostridium difficile Toxin B, whereas basolateral infection of incompletely polarized MDCK monolayers was more efficient and required activation of Rho-family GTPases. Together, our findings suggest that as epithelial barrier differentiates and becomes highly polarized, it becomes resistant to P. aeruginosa infection. Nevertheless, polarized epithelial cells still sense the presence of apically infecting P. aeruginosa, but they may do so through a different group of surface proteins and/or downstream signaling pathways than do incompletely polarized cells.
Figures
Similar articles
-
Rho GTPase activity modulates Pseudomonas aeruginosa internalization by epithelial cells.Cell Microbiol. 2001 Feb;3(2):85-98. doi: 10.1046/j.1462-5822.2001.00091.x. Cell Microbiol. 2001. PMID: 11207623
-
Modification of epithelial cell barrier permeability and intercellular junctions by Clostridium sordellii lethal toxins.Cell Microbiol. 2006 Jul;8(7):1070-85. doi: 10.1111/j.1462-5822.2006.00687.x. Cell Microbiol. 2006. PMID: 16819961
-
Epithelial cell polarity affects susceptibility to Pseudomonas aeruginosa invasion and cytotoxicity.Infect Immun. 1997 Jul;65(7):2861-7. doi: 10.1128/iai.65.7.2861-2867.1997. Infect Immun. 1997. PMID: 9199460 Free PMC article.
-
Epithelial junctions and Rho family GTPases: the zonular signalosome.Small GTPases. 2014;5(4):1-15. doi: 10.4161/21541248.2014.973760. Small GTPases. 2014. PMID: 25483301 Free PMC article. Review.
-
The interdependence of the Rho GTPases and apicobasal cell polarity.Small GTPases. 2014;5(2):10. doi: 10.4161/21541248.2014.973768. Small GTPases. 2014. PMID: 25469537 Free PMC article. Review.
Cited by
-
Pseudomonas aeruginosa exploits a PIP3-dependent pathway to transform apical into basolateral membrane.J Cell Biol. 2007 Apr 9;177(1):21-7. doi: 10.1083/jcb.200605142. Epub 2007 Apr 2. J Cell Biol. 2007. PMID: 17403925 Free PMC article.
-
Subversion of mucosal barrier polarity by pseudomonas aeruginosa.Front Microbiol. 2011 May 26;2:114. doi: 10.3389/fmicb.2011.00114. eCollection 2011. Front Microbiol. 2011. PMID: 21747810 Free PMC article.
-
RNAi screen reveals an Abl kinase-dependent host cell pathway involved in Pseudomonas aeruginosa internalization.PLoS Pathog. 2008 Mar 21;4(3):e1000031. doi: 10.1371/journal.ppat.1000031. PLoS Pathog. 2008. PMID: 18369477 Free PMC article.
-
Pseudomonas aeruginosa survives in epithelia by ExoS-mediated inhibition of autophagy and mTOR.EMBO Rep. 2021 Feb 3;22(2):e50613. doi: 10.15252/embr.202050613. Epub 2020 Dec 20. EMBO Rep. 2021. PMID: 33345425 Free PMC article.
-
PAK in pathogen-host interactions.Cell Logist. 2012 Apr 1;2(2):126-131. doi: 10.4161/cl.20222. Cell Logist. 2012. PMID: 23125952 Free PMC article.
References
-
- Anastasiadis, P.Z., Moon, S.Y., Thoreson, M.A., Mariner, D.J., Crawford, H.C., Zheng, Y., and Reynolds, A.B. (2000). Inhibition of RhoA by p120 catenin. Nat. Cell Biol. 2, 637-644. - PubMed
-
- Bagrodia, S., Taylor, S.J., Jordon, K.A., Van Aelst, L., and Cerione, R.A. (1998). A novel regulator of p21-activated kinases. J. Biol. Chem. 273, 23633-23636. - PubMed
-
- Benard, V., Bohl, B.P., and Bokoch, G.M. (1999). Characterization of Rac and Cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J. Biol. Chem. 274, 13198-13204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

