Novel membrane protein shrew-1 targets to cadherin-mediated junctions in polarized epithelial cells
- PMID: 14595118
- PMCID: PMC307556
- DOI: 10.1091/mbc.e03-05-0281
Novel membrane protein shrew-1 targets to cadherin-mediated junctions in polarized epithelial cells
Abstract
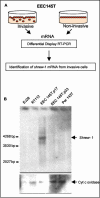
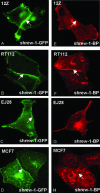
While searching for potential candidate molecules relevant for the pathogenesis of endometriosis, we discovered a 2910-base pair cDNA encoding a novel putative 411-amino acid integral membrane protein that we called shrew-1. The putative open-reading frame was confirmed with antibodies against shrew-1 peptides that labeled a protein of approximately 48 kDa in extracts of shrew-1 mRNA-positive tissue and also detected ectopically expressed shrew-1. Expression of epitope-tagged shrew-1 in epithelial cells and analysis by surface biotinylation and immunoblots demonstrated that shrew-1 is indeed a transmembrane protein. Shrew-1 is able to target to E-cadherin-mediated adherens junctions and interact with the E-cadherin-catenin complex in polarized MCF7 and Madin-Darby canine kidney cells, but not with the N-cadherin-catenin complex in nonpolarized epithelial cells. Direct interaction of shrew-1 with beta-catenin in in vitro pull-down assay suggests that beta-catenin might be one of the proteins that targets and/or retains shrew-1 in the adherens junctions. Interestingly, shrew-1 was partially translocated in response to scatter factor (ligand of receptor tyrosine kinase c-met) from the plasma membrane to the cytoplasm where it still colocalized with endogenous E-cadherin. In summary, we introduce shrew-1 as a novel component of adherens junctions, interacting with E-cadherin-beta-catenin complexes in polarized epithelial cells.
Figures






References
-
- Aberle, H., Butz, S., Stappert, J., Weissig, H., Kemler, R., and Hoschuetzky, H. (1994). Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J. Cell Sci. 107, 3655-3663. - PubMed
-
- Aberle, H., Schwartz, H., Hoschuetzky, H., and Kemler, R. (1996). Single amino acid substitutions in proteins of the armadillo gene family abolish their binding to alpha-catenin. J. Biol. Chem. 271, 1520-1526. - PubMed
-
- Anastasiadis, P.Z., Moon, S.Y., Thoreson, M.A., Mariner, D.J., Crawford, H.C., Zheng, Y., and Reynolds, A.B. (2000). Inhibition of RhoA by p120 catenin. Nat. Cell Biol. 2, 637-644. - PubMed
-
- Birchmeier, W., Weidner, K.M., and Behrens, J. (1993). Molecular mechanisms leading to loss of differentiation and gain of invasiveness in epithelial cells. J. Cell Sci. Suppl. 17, 159-164. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

