An mRNA structure in bacteria that controls gene expression by binding lysine
- PMID: 14597663
- PMCID: PMC280618
- DOI: 10.1101/gad.1140003
An mRNA structure in bacteria that controls gene expression by binding lysine
Abstract
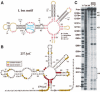
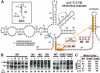
Riboswitches are metabolite-responsive genetic control elements that reside in the untranslated regions (UTRs) of certain messenger RNAs. Herein, we report that the 5'-UTR of the lysC gene of Bacillus subtilis carries a conserved RNA element that serves as a lysine-responsive riboswitch. The ligand-binding domain of the riboswitch binds to L-lysine with an apparent dissociation constant (KD) of approximately 1 micro M, and exhibits a high level of molecular discrimination against closely related analogs, including D-lysine and ornithine. Furthermore, we provide evidence that this widespread class of riboswitches serves as a target for the antimetabolite S-(2-aminoethyl)-L-cysteine (AEC). These findings add support to the hypotheses that direct sensing of metabolites by messenger RNAs is a fundamental form of genetic control and that riboswitches represent a new class of antimicrobial drug targets.
Figures



References
-
- Belitsky B.R. 2002. Biosynthesis of amino acids of the glutamate and aspartate families, alanine, and polyamines. In Bacillus subtilis and its closest relatives: From genes to cells. (eds. A.L. Sonenshein, et al.), pp. 203-231. ASM Press, Washington, D.C.
-
- Boy E., Borne, F., and Patte, J.C. 1979. Isolation and identification of mutants constitutive for aspartokinase III synthesis in Escherichia coli K12. Biochimie 61: 1151-1160. - PubMed
-
- Di Girolamo M., Busiello, V., Coccia, R., and Foppoli, C. 1990. Aspartokinase III repression and lysine analogs utilization for protein synthesis. Physiol. Chem. Phys. Med. NMR 22: 241-245. - PubMed
-
- Dong Z. 1992. An efficient asymmetric synthesis of L-α-ω-diaminoalkanoic acids. Tetrahedron Lett. 33: 7725-7726.
-
- Engel M.H. and Hare, P.E. 1982. Racemization rates of the basic amino acids. Year Book Carnegie Inst. Washington 81: 422-425.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
