Aquaporin-4 square array assembly: opposing actions of M1 and M23 isoforms
- PMID: 14597700
- PMCID: PMC263861
- DOI: 10.1073/pnas.2235843100
Aquaporin-4 square array assembly: opposing actions of M1 and M23 isoforms
Abstract
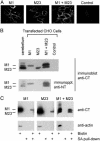
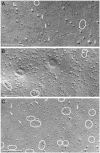
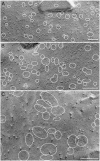
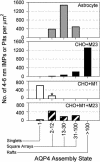
Osmotic homeostasis in the brain involves movement of water through aquaporin-4 (AQP4) membrane channels. Perivascular astrocyte end-feet contain distinctive orthogonal lattices (square arrays) assembled from 4- to 6-nm intramembrane particles (IMPs) corresponding to individual AQP4 tetramers. Two isoforms of AQP4 result from translation initiation at methionine residues M1 and M23, but no functional differences are known. In this study, Chinese hamster ovary cells were transfected with M1, M23, or M1+M23 isoforms, and AQP4 expression was confirmed by immunoblotting, immunocytochemistry, and immunogold labeling. Square array organization was examined by freeze-fracture electron microscopy. In astrocyte end-feet, >90% of 4- to 6-nm IMPs were found in square arrays, with 65% in arrays of 13-30 IMPs. In cells transfected with M23, 95% of 4- to 6-nm IMPs were in large assemblies (rafts), 85% of which contained >100 IMPs. However, in M1 cells, >95% of 4- to 6-nm IMPs were present as singlets, with <5% in incipient arrays of 2-12 IMPs. In M1+M23 cells, 4- to 6-nm IMPs were in arrays of intermediate sizes, resembling square arrays in astrocytes. Structural cross-bridges of 1 x 2 nm linked >90% of IMPs in M23 arrays ( approximately 1,000 cross-bridges per microm2) but were rarely seen in M1 cells. These studies show that M23 and M1 isoforms have opposing effects on intramembrane organization of AQP4: M23 forms large square arrays with abundant cross-bridges; M1 restricts square array assembly.
Figures



References
-
- Neely, J. D., Christensen, B. M., Nielsen, S. & Agre, P. (1999) Biochemistry 38, 11156-11163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL33991/HL/NHLBI NIH HHS/United States
- R01 EY011239/EY/NEI NIH HHS/United States
- R01 NS038121/NS/NINDS NIH HHS/United States
- R37 HL048268/HL/NHLBI NIH HHS/United States
- R01 HL033991/HL/NHLBI NIH HHS/United States
- NS-44395/NS/NINDS NIH HHS/United States
- R01 HL048268/HL/NHLBI NIH HHS/United States
- R01 NS044395/NS/NINDS NIH HHS/United States
- HL-48268/HL/NHLBI NIH HHS/United States
- NS-38121/NS/NINDS NIH HHS/United States
- R01 NS044010/NS/NINDS NIH HHS/United States
- NS-44010/NS/NINDS NIH HHS/United States
- R56 NS044395/NS/NINDS NIH HHS/United States
- EY-11239/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

