Delayed K+ clearance associated with aquaporin-4 mislocalization: phenotypic defects in brains of alpha-syntrophin-null mice
- PMID: 14597704
- PMCID: PMC263862
- DOI: 10.1073/pnas.2336064100
Delayed K+ clearance associated with aquaporin-4 mislocalization: phenotypic defects in brains of alpha-syntrophin-null mice
Abstract
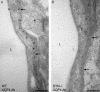
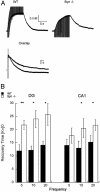
Recovery from neuronal activation requires rapid clearance of potassium ions (K+) and restoration of osmotic equilibrium. The predominant water channel protein in brain, aquaporin-4 (AQP4), is concentrated in the astrocyte end-feet membranes adjacent to blood vessels in neocortex and cerebellum by association with alpha-syntrophin protein. Although AQP4 has been implicated in the pathogenesis of brain edema, its functions in normal brain physiology are uncertain. In this study, we used immunogold electron microscopy to compare hippocampus of WT and alpha-syntrophin-null mice (alpha-Syn-/-). We found that <10% of AQP4 immunogold labeling is retained in the perivascular astrocyte end-feet membranes of the alpha-Syn-/- mice, whereas labeling of the inwardly rectifying K+ channel, Kir4.1, is largely unchanged. Activity-dependent changes in K+ clearance were studied in hippocampal slices to test whether AQP4 and K+ channels work in concert to achieve isosmotic clearance of K+ after neuronal activation. Microelectrode recordings of extracellular K+ ([K+]o) from the target zones of Schaffer collaterals and perforant path were obtained after 5-, 10-, and 20-Hz orthodromic stimulations. K+ clearance was prolonged up to 2-fold in alpha-Syn-/- mice compared with WT mice. Furthermore, the intensity of hyperthermia-induced epileptic seizures was increased in approximately half of the alpha-Syn-/-mice. These studies lead us to propose that water flux through perivascular AQP4 is needed to sustain efficient removal of K+ after neuronal activation.
Figures
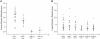

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

