Endosomal compartmentalization in three dimensions: implications for membrane fusion
- PMID: 14597718
- PMCID: PMC263806
- DOI: 10.1073/pnas.2232379100
Endosomal compartmentalization in three dimensions: implications for membrane fusion
Abstract
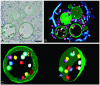
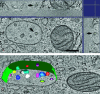
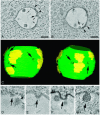
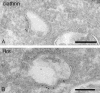
Endosomes are major sorting stations in the endocytic route that send proteins and lipids to multiple destinations in the cell, including the cell surface, Golgi complex, and lysosomes. They have an intricate architecture of internal membrane structures enclosed by an outer membrane. Recycling proteins remain on the outer membrane, whereas proteins that are destined for degradation in the lysosome are sorted to the interior. Recently, a retrograde pathway was discovered whereby molecules, like MHC class II of the immune system, return from the internal structures to the outer membrane, allowing their further transport to the cell surface for T cell activation. Whether this return involves back fusion of free vesicles with the outer membrane, or occurs via the continuity of the two membrane domains, is an unanswered question. By electron tomography of cryo-immobilized cells we now demonstrate that, in multivesicular endosomes of B-lymphocytes and dendritic cells, the inner membranes are free vesicles. Hence, protein transport from inner to outer membranes cannot occur laterally in the plane of the membrane, but requires fusion between the two membrane domains. This implies the existence of an intracellular machinery that mediates fusion between the exoplasmic leaflets of the membranes involved, which is opposite to regular intracellular fusion between cytoplasmic leaflets. In addition, our 3D reconstructions reveal the presence of clathrin-coated areas at the cytoplasmic face of the outer membrane, known to participate in protein sorting to the endosomal interior. Interestingly, profiles reminiscent of inward budding vesicles were often in close proximity to the coats.
Figures

References
-
- Mellman, I. (1996) Annu. Rev. Cell Dev. Biol. 12, 575-625. - PubMed
-
- Stoorvogel, W., Strous, G. J., Geuze, H. J., Oorschot, V. & Schwartz, A. L. (1991) Cell 65, 417-427. - PubMed
-
- Raposo, G., Vidal, M. & Geuze, H. J. (1997) in Unusual Secretory Pathways: From Batceria to Man, eds. Kuchler, K., Rubartelli, A. & Holland, B. (R. G. Landes, Washington, DC), pp. 161-184.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

