Pannexins, a family of gap junction proteins expressed in brain
- PMID: 14597722
- PMCID: PMC263867
- DOI: 10.1073/pnas.2233464100
Pannexins, a family of gap junction proteins expressed in brain
Abstract
Database search has led to the identification of a family of proteins, the pannexins, which share some structural features with the gap junction forming proteins of invertebrates and vertebrates. The function of these proteins has remained unclear so far. To test the possibility that pannexins underlie electrical communication in the brain, we have investigated their tissue distribution and functional properties. Here, we show that two of these genes, pannexin 1 (Px1) and Px2, are abundantly expressed in the CNS. In many neuronal cell populations, including hippocampus, olfactory bulb, cortex and cerebellum, there is coexpression of both pannexins, whereas in other brain regions, e.g., white matter, only Px1-positive cells were found. On expression in Xenopus oocytes, Px1, but not Px2 forms functional hemichannels. Coinjection of both pannexin RNAs results in hemichannels with functional properties that are different from those formed by Px1 only. In paired oocytes, Px1, alone and in combination with Px2, induces the formation of intercellular channels. The functional characteristics of homomeric Px1 versus heteromeric Px1/Px2 channels and the different expression patterns of Px1 and Px2 in the brain indicate that pannexins form cell type-specific gap junctions with distinct properties that may subserve different functions.
Figures
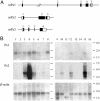

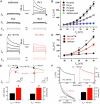

References
-
- Willecke, K., Eiberger, J., Degen, J., Eckardt, D., Romualdi, A., Guldenagel, M., Deutsch, U. & Sohl, G. (2002) Biol. Chem. 383, 725-737. - PubMed
-
- Bennett, M. V. (2000) Brain Res. Brain Res. Rev. 32, 16-28. - PubMed
-
- Galarreta, M. & Hestrin, S. (2001) Nat. Rev. Neurosci. 2, 425-433. - PubMed
-
- Katsumaru, H., Kosaka, T., Heizmann, C. W. & Hama, K. (1988) Exp. Brain Res. 72, 363-370. - PubMed
-
- Galarreta, M. & Hestrin, S. (1999) Nature 402, 72-75. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

