A novel endothelial L-selectin ligand activity in lymph node medulla that is regulated by alpha(1,3)-fucosyltransferase-IV
- PMID: 14597733
- PMCID: PMC2194247
- DOI: 10.1084/jem.20030182
A novel endothelial L-selectin ligand activity in lymph node medulla that is regulated by alpha(1,3)-fucosyltransferase-IV
Abstract
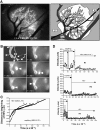
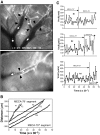
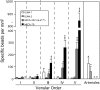
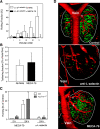
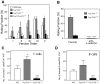
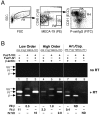
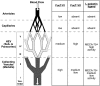
Lymphocytes home to peripheral lymph nodes (PLNs) via high endothelial venules (HEVs) in the subcortex and incrementally larger collecting venules in the medulla. HEVs express ligands for L-selectin, which mediates lymphocyte rolling. L-selectin counterreceptors in HEVs are recognized by mAb MECA-79, a surrogate marker for molecularly heterogeneous glycans termed peripheral node addressin. By contrast, we find that medullary venules express L-selectin ligands not recognized by MECA-79. Both L-selectin ligands must be fucosylated by alpha(1,3)-fucosyltransferase (FucT)-IV or FucT-VII as rolling is absent in FucT-IV+VII(-/-) mice. Intravital microscopy experiments revealed that MECA-79-reactive ligands depend primarily on FucT-VII, whereas MECA-79-independent medullary L-selectin ligands are regulated by FucT-IV. Expression levels of both enzymes paralleled these anatomical distinctions. The relative mRNA level of FucT-IV was higher in medullary venules than in HEVs, whereas FucT-VII was most prominent in HEVs and weak in medullary venules. Thus, two distinct L-selectin ligands are segmentally confined to contiguous microvascular domains in PLNs. Although MECA-79-reactive species predominate in HEVs, medullary venules express another ligand that is spatially, antigenically, and biosynthetically unique. Physiologic relevance for this novel activity in medullary microvessels is suggested by the finding that L-selectin-dependent T cell homing to PLNs was partly insensitive to MECA-79 inhibition.
Figures
Comment in
-
Sulfated sugars for rolling lymphocytes.J Exp Med. 2003 Nov 3;198(9):1285-8. doi: 10.1084/jem.20031664. J Exp Med. 2003. PMID: 14597731 Free PMC article. No abstract available.
References
-
- von Andrian, U.H., and C.R. Mackay. 2000. T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 343:1020–1034. - PubMed
-
- Springer, T.A. 1994. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multi-step paradigm. Cell. 76:301–314. - PubMed
-
- Stein, J.V., A. Rot, Y. Luo, M. Narasimhaswamy, H. Nakano, M.D. Gunn, A. Matsuzawa, E.J. Quackenbush, M.E. Dorf, and U.H. von Andrian. 2000. The CC chemokine thymus-derived chemotactic agent 4 (TCA-4, secondary lymphoid tissue chemokine, 6Ckine, exodus-2) triggers lymphocyte function–associated antigen 1–mediated arrest of rolling T lymphocytes in peripheral lymph node high endothelial venules. J. Exp. Med. 191:61–76. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

