CCR5 expression influences the progression of human breast cancer in a p53-dependent manner
- PMID: 14597737
- PMCID: PMC2194244
- DOI: 10.1084/jem.20030580
CCR5 expression influences the progression of human breast cancer in a p53-dependent manner
Abstract
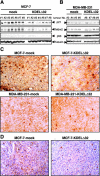
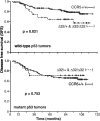
Chemokines are implicated in tumor pathogenesis, although it is unclear whether they affect human cancer progression positively or negatively. We found that activation of the chemokine receptor CCR5 regulates p53 transcriptional activity in breast cancer cells through pertussis toxin-, JAK2-, and p38 mitogen-activated protein kinase-dependent mechanisms. CCR5 blockade significantly enhanced proliferation of xenografts from tumor cells bearing wild-type p53, but did not affect proliferation of tumor xenografts bearing a p53 mutation. In parallel, data obtained in a primary breast cancer clinical series showed that disease-free survival was shorter in individuals bearing the CCR5Delta32 allele than in CCR5 wild-type patients, but only for those whose tumors expressed wild-type p53. These findings suggest that CCR5 activity influences human breast cancer progression in a p53-dependent manner.
Figures



References
-
- Murphy, P. 2001. Chemokines and molecular basis of cancer metastasis. N. Engl. J. Med. 354:833–835. - PubMed
-
- Balkwill, F., and A. Mantovani. 2001. Inflammation and cancer: back to Virchow? Lancet. 357:539–545. - PubMed
-
- Homey, B., A. Muller, and A. Zlotnik. 2002. Chemokines: agents for the immunotherapy of cancer? Nat. Rev. Immunol. 2:175–184. - PubMed
-
- Mashino, K., N. Sadanaga, H. Yamaguchi, F. Tanaka, M. Ohta, K. Shibuta, H. Inoue, and M. Mori. 2002. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res. 62:2937–2941. - PubMed
-
- Muller, A., B. Homey, H. Soto, N. Ge, D. Catron, M. Buchanan, T. McClanahan, E. Murphy, W. Yuan, S. Wagner, et al. 2001. Involvement of chemokine receptors in breast cancer metastasis. Nature. 410:50–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

