Age-dependent requirement for gammadelta T cells in the primary but not secondary protective immune response against an intestinal parasite
- PMID: 14597739
- PMCID: PMC2194243
- DOI: 10.1084/jem.20030050
Age-dependent requirement for gammadelta T cells in the primary but not secondary protective immune response against an intestinal parasite
Abstract
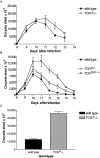
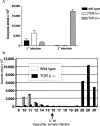
Between weaning (3 wk of age) and adulthood (7 wk of age), mice develop increased resistance to infection with Eimeria vermiformis, an abundant intestinal parasite that causes coccidiosis. This development of resistance was perturbed in T cell receptor (TCR)delta(-/-) mice, which at 4 wk of age remained largely susceptible to infection and prone to infection-associated dehydration. These phenotypes were rescued by the repopulation of gammadelta cells after adoptive transfer of lymphoid progenitors into newborn recipients. Because alphabeta T cells are necessary and sufficient for the protection of adult mice against E. vermiformis, the requirement for gammadelta cells in young mice shows a qualitative difference between the cellular immune responses operating at different ages. An important contribution toward primary immune protection in young hosts may have provided a strong selective pressure for the evolutionary conservation of gammadelta cells. This notwithstanding, the development of effective, pathogen-specific immunity in young mice requires alphabeta T cells, just as it does in adult mice.
Figures





References
-
- Adkins, B. 2000. Development of neonatal Th1/Th2 function. Int. Rev. Immunol. 19:157–171. - PubMed
-
- Marshall-Clarke, S., D. Reen, L. Tasker, and J. Hassan. 2000. Neonatal immunity: how well has it grown up? Immunol. Today. 21:35–41. - PubMed
-
- Cuisinier, A.M., F. Fumoux, D. Moinier, L. Boubli, V. Guigou, M. Milili, C. Schiff, M. Fougereau, and C. Tonnelle. 1990. Rapid expansion of human immunoglobulin repertoire (VH, V kappa, V lambda) expressed in early fetal bone marrow. New Biol. 2:689–699. - PubMed
-
- Billingham, R., L. Brent, and P.B. Medawar. 1953. Actively acquired tolerance of foreign cells. Nature. 172:603–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

