Interspecies communication in bacteria
- PMID: 14597753
- PMCID: PMC228483
- DOI: 10.1172/JCI20195
Interspecies communication in bacteria
Abstract
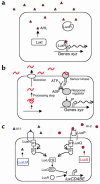
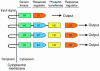
Until recently, bacteria were considered to live rather asocial, reclusive lives. New research shows that, in fact, bacteria have elaborate chemical signaling systems that enable them to communicate within and between species. One signal, termed AI-2, appears to be universal and facilitates interspecies communication. Many processes, including virulence factor production, biofilm formation, and motility, are controlled by AI-2. Strategies that interfere with communication in bacteria are being explored in the biotechnology industry with the aim of developing novel antimicrobials. AI-2 is a particularly attractive candidate for such studies because of its widespread use in the microbial kingdom.
Figures

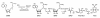
References
-
- Miller MB, Bassler BL. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001;55:165–199. - PubMed
-
- Engebrecht J, Nealson K, Silverman M. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell. 1983;32:773–781. - PubMed
-
- Lazazzera BA, Grossman AD. The ins and outs of peptide signaling. Trends Microbiol. 1998;6:288–294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

