Angiotensin II-accelerated atherosclerosis and aneurysm formation is attenuated in osteopontin-deficient mice
- PMID: 14597759
- PMCID: PMC228408
- DOI: 10.1172/JCI18141
Angiotensin II-accelerated atherosclerosis and aneurysm formation is attenuated in osteopontin-deficient mice
Abstract
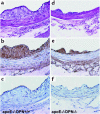
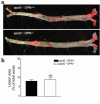
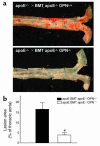
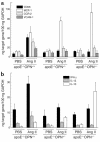
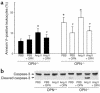
Osteopontin (OPN) is expressed in atherosclerotic lesions, particularly in diabetic patients. To determine the role of OPN in atherogenesis, ApoE-/-OPN+/+, ApoE-/-OPN+/-, and ApoE-/-OPN-/- mice were infused with Ang II, inducing vascular OPN expression and accelerating atherosclerosis. Compared with ApoE-/-OPN+/+ mice, ApoE-/-OPN+/- and ApoE-/-OPN-/- mice developed less Ang II-accelerated atherosclerosis. ApoE-/- mice transplanted with bone marrow derived from ApoE-/-OPN-/- mice had less Ang II-induced atherosclerosis compared with animals receiving ApoE-/-OPN+/+ cells. Aortae from Ang II-infused ApoE-/-OPN-/- mice expressed less CD68, C-C-chemokine receptor 2, and VCAM-1. In response to intraperitoneal thioglycollate, recruitment of leukocytes in OPN-/- mice was impaired, and OPN-/- leukocytes exhibited decreased basal and MCP-1-directed migration. Furthermore, macrophage viability in atherosclerotic lesions from Ang II-infused ApoE-/-OPN-/- mice was decreased. Finally, Ang II-induced abdominal aortic aneurysm formation in ApoE-/-OPN-/- mice was reduced and associated with decreased MMP-2 and MMP-9 activity. These data suggest an important role for leukocyte-derived OPN in mediating Ang II-accelerated atherosclerosis and aneurysm formation.
Figures


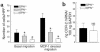


References
-
- Denhardt DT, Giachelli CM, Rittling SR. Role of osteopontin in cellular signaling and toxicant injury. Annu. Rev. Pharmacol. Toxicol. 2001;41:723–749. - PubMed
-
- Katayama Y, et al. Casein kinase 2 phosphorylation of recombinant rat osteopontin enhances adhesion of osteoclasts but not osteoblasts. J. Cell Physiol. 1998;176:179–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

