Inhibition of endogenous thioredoxin in the heart increases oxidative stress and cardiac hypertrophy
- PMID: 14597765
- PMCID: PMC228400
- DOI: 10.1172/JCI17700
Inhibition of endogenous thioredoxin in the heart increases oxidative stress and cardiac hypertrophy
Abstract
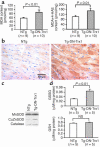
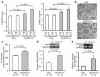
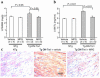
Thioredoxin 1 (Trx1) has redox-sensitive cysteine residues and acts as an antioxidant in cells. However, the extent of Trx1 contribution to overall antioxidant mechanisms is unknown in any organs. We generated transgenic mice with cardiac-specific overexpression of a dominant negative (DN) mutant (C32S/C35S) of Trx1 (Tg-DN-Trx1 mice), in which the activity of endogenous Trx was diminished. Markers of oxidative stress were significantly increased in hearts from Tg-DN-Trx1 mice compared with those from nontransgenic (NTg) mice. Tg-DN-Trx1 mice exhibited cardiac hypertrophy with maintained cardiac function at baseline. Intraperitoneal injection of N-2-mercaptopropionyl glycine, an antioxidant, normalized cardiac hypertrophy in Tg-DN-Trx1 mice. Thoracic aortic banding caused greater increases in myocardial oxidative stress and enhanced hypertrophy in Tg-DN-Trx1 compared with NTg mice. In contrast, transgenic mice with cardiac-specific overexpression of wild-type Trx1 did not show cardiac hypertrophy at baseline but exhibited reduced levels of hypertrophy and oxidative stress in response to pressure overload. These results demonstrate that endogenous Trx1 is an essential component of the cellular antioxidant mechanisms and plays a critical role in regulating oxidative stress in the heart in vivo. Furthermore, inhibition of endogenous Trx1 in the heart primarily stimulates hypertrophy, both under basal conditions and in response to pressure overload through redox-sensitive mechanisms.
Figures

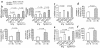



References
-
- Sawyer DB, et al. Role of oxidative stress in myocardial hypertrophy and failure. J. Mol. Cell. Cardiol. 2002;34:379–388. - PubMed
-
- Sorescu D, Griendling KK. Reactive oxygen species, mitochondria, and NAD(P)H oxidases in the development and progression of heart failure. Congest. Heart Fail. 2002;8:132–140. - PubMed
-
- Nakamura H, Nakamura K, Yodoi J. Redox regulation of cellular activation. Annu. Rev. Immunol. 1997;15:351–369. - PubMed
-
- Masutani H, Yodoi J. Thioredoxin. Overview. Methods Enzymol. 2002;347:279–286. - PubMed
-
- Kishimoto C, et al. Serum thioredoxin (TRX) levels in patients with heart failure. Jpn. Circ. J. 2001;65:491–494. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 HL033107/HL/NHLBI NIH HHS/United States
- HL-67727/HL/NHLBI NIH HHS/United States
- HL-67724/HL/NHLBI NIH HHS/United States
- HL-69020/HL/NHLBI NIH HHS/United States
- R01 HL033107/HL/NHLBI NIH HHS/United States
- AG-14121/AG/NIA NIH HHS/United States
- HL-33107/HL/NHLBI NIH HHS/United States
- HL-59139/HL/NHLBI NIH HHS/United States
- HL-65183/HL/NHLBI NIH HHS/United States
- P01 HL069020/HL/NHLBI NIH HHS/United States
- HL-33065/HL/NHLBI NIH HHS/United States
- R01 HL067724/HL/NHLBI NIH HHS/United States
- R01 HL065182/HL/NHLBI NIH HHS/United States
- P01 HL059139/HL/NHLBI NIH HHS/United States
- HL-65182/HL/NHLBI NIH HHS/United States
- R01 AG014121/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

